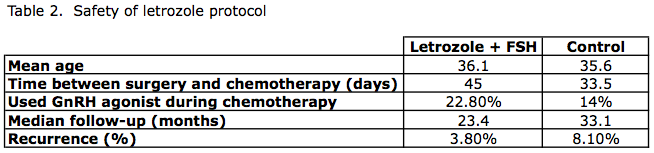Additional Considerations for COS in Cancer Patients: Safety
| Is COS safe in women with cancer? |
There is little published data about the safety of a COS to bank embryos or oocytes prior to cancer treatments. Hypothetical risks include:
The risk of delaying cancer treatments to undergo a COS cycle
- Several researchers have published reassuring data, demonstrating that cancer treatments are not significantly delayed2,3.
- Baynosa et al. evaluated this concern and did not demonstrate a delay in the start of chemotherapy, specifically in the breast cancer population2. The median time from definitive surgery to chemotherapy was 30 days (range 12-100 days) in women who underwent COS compared to 29 days (range 12 to 120 days) in women who did not.
- In a study of 205 COS cycles for women with a variety of cancer types, the FertiPROTEKT network reports that “none of the patients needed to have the start of their chemotherapy postponed beyond the time that had been planned for ovarian stimulation”3.
The risk that COS may itself increase the risk of cancer recurrence
- This may be even more of a concern in women with hormone-sensitive cancers.
- The only published study evaluating cancer recurrence looks specifically at breast cancer patients using the letrozole protocol4.
Routine IVF risks may be heightened in a sicker cancer population
- Ovarian hyperstimulation syndrome (OHSS). Using a GnRHa trigger, instead of an HCG trigger, results in a lower incidence of OHSS and a more rapid drop in post-retrieval estrogen levels5. For example, many centers use Leuprolide acetate, 1-2 mg SQ.
- Oocyte retrieval procedure
- Coagulopathy, clotting risks
- Infection risk
|
| Is COH safe in women with hormone-sensitive cancers? |
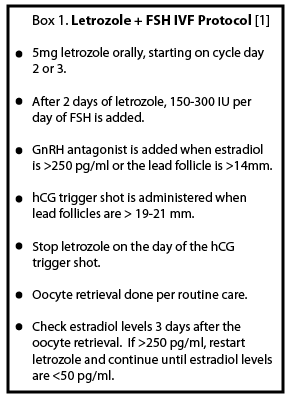 Background about the letrozole protocol Background about the letrozole protocol
- With the goal of mitigating the peak estradiol levels during stimulation, Oktay et al. developed a modified protocol using an anti-cancer hormonal therapy during an “Antagonist Cycle” in women with hormone-sensitive breast cancer1,3.
- Various strategies to decrease peak estrogen levels have been employed, using either aromatase inhibitors or anti-estrogens. In a non-randomized trial looking at IVF cycles with gonadotropins plus either tamoxifen or letrozole, IVF outcomes were similar, but the peak estradiol levels were significantly lower with the letrozole treatment1. Another study looked at the aromatase-inhibitor anastrozole, which has been shown to be 2.5 times more potent at suppressing estrogen levels than letrozole6. However, interestingly, in combination with gonadotropins in an IVF cycle, overall estradiol levels were higher in women on anastrozole versus letrozole4.
- Therefore, in women with hormone-sensitive breast cancer, the recent focus has been COS with a combination of letrozole and gonadotropins.
- Subsequent studies have been published that are reassuring regarding IVF outcomes and safety of this protocol.
- In addition, one study used this protocol in women with another hormone-sensitive cancer, endometrial carcinoma7.
Letrozole protocol: IVF outcomes
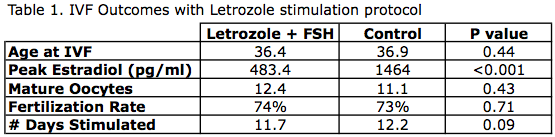
Oktay et al.1 performed a retrospective analysis of IVF cycles in women with hormone sensitive cancers, looking at outcomes such as oocyte yield, peak estradiol levels, and time to chemotherapy start (Table 1).
- 47 women with Stage I-IIIa breast cancer
- IVF protocol with letrozole (see Box 1)
- Controls: Age-matched women undergoing IVF for tubal factor infertility
- Conclusions:
- No difference in the number of mature oocytes, fertilization rate, and number of days stimulated
- Significantly lower peak estradiol levels and less gonadotropin medications needed
Letrozole Protocol: Safety
Azim et al.4 followed women who underwent this modified IVF protocol using letrozole prospectively, and reported on recurrence rates over the next 2-3 years. (Table 2)
- Prospective evaluation
- Non-randomized study
- 215 women with breast cancer
- Cases: 79 patients – IVF (letrozole and FSH)
- Controls: 136 patients – no ovarian stimulation done
- Mean follow up: 23.4 months in IVF group, 33 months in controls
Conclusions: No difference in recurrence rates of breast cancer in women who underwent a letrozole stimulation protocol, compared to women who did not.
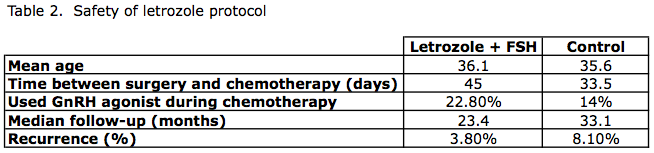
|
References
1. Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab 2006;91:3885-90.
2. Baynosa J, Westphal LM, Madrigrano A, Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg 2009; 209: 603-7.
3. Lawrenz B, Jauckus J, Kupka M, Strowitzki T, con Wolff M. Efficacy and safety of ovarian stimulation before chemotherapy in 205 cases. Fertil Steril 2010; 94: 2871-3.
4. Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol 2008;26:2630-5.
5. Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online; 20:738-8.
6. Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 2002;20:751-7.
7. Azim A, Oktay K. Letrozole for ovulation induction and fertility preservation by embryo cryopreservation in young women with endometrial carcinoma. Fertil Steril 2007;88:657-64.
About the Author
Jennifer Mersereau, MD, MSCI, is an reproductive endocrinologist in the University of North Carolina’s Department of Obstetrics and Gynecology. As the Director of the Fertility Preservation Program, she has extensive experience guiding patients and physicians through the oncofertility experience.
Page last updated March 14, 2012.
 Background about the letrozole protocol
Background about the letrozole protocol