Today we welcome Dr. So-Youn Kim from Dr. Woodruff’s Lab at Northwestern University as our guest blogger. Dr. Kim will review a recent article published by Dr. John C. Schimenti in Science 2014.
ONE MORE STEP TOWARDS PRESERVING OOCYTE RESERVE
By: So-Youn Kim, PhD
According to a paper published by Dr. John C. Schimenti and colleagues in Science in 2014, Reversal of Female Infertility by Chk2 Ablation Reveals the Oocyte DNA Damage Checkpoint Pathway, mice that have knock out checkpoint kinase 2 (chek2-/- or chk2-/-) are protected against radiation damage to oocytes (1). These Chk2-deficient female mice do not have fertility problems. Radiation did not eliminate primordial follicles in Chk2-deficient female ovaries, compared with that of wild-type animals, and Chk2-deficient mice gave birth to an average litter size. This gives researchers further hope that oocytes can be protected during chemo- or radiation therapy in cancer patients.
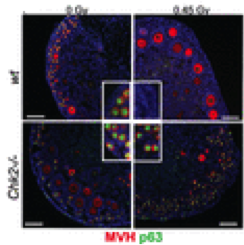 Depletion of p63-positive primordial follicles by IR is CHK2-dependent. Ovaries were cultured 7 days after irradiation. Scale bars, 100 μm. MVH marks oocytes. (Insets) Ovary cortical regions containing primordial follicles (Schimenti J.C. et al, Science, 2014)
Depletion of p63-positive primordial follicles by IR is CHK2-dependent. Ovaries were cultured 7 days after irradiation. Scale bars, 100 μm. MVH marks oocytes. (Insets) Ovary cortical regions containing primordial follicles (Schimenti J.C. et al, Science, 2014)Checkpoint kinase 2 (CHK2) is a protein component of the meiotic DNA damage checkpoint that plays a role in DNA repair. It is a downstream molecule of ataxia telangiectasia mutated (ATM), which is activated to DNA double strand breaks (DSBs), or ataxia telangiectasia and Rad3 related (ATR; FRP1), which is responsive mainly to single-stranded DNA breaks (2,3). CHK2 may transfer the signal to downstream molecules, p63/p53 (/p73), to decide the destiny of cells. Schimenti’s team showed that irradiated Chk2-/- mice did not phosphorylate TAp63, a protein that has known as a guardian in oocyte to protect female germline (4). The team showed that CHK2 signals to both p53 and TAp63 when oocytes get DSBs by showing that irradiated p53-/- TAp63-/- has similar protection of Chk2-/- female. Therefore, if the repair systems by CHK2 fail to fix DNA damage, it will induce the signaling pathway of p53/TAp63, causing the depletion of ovarian follicle reserve.
Women who are treated with chemo- or radiation therapy are faced with the depletion of ovarian reserve, causing premature ovarian failure and destruction of the endocrine system. Chemo- or radiation therapy is especially detrimental to women who have not yet given birth, but hope to have children later in their life.
Many groups have done research to protect oocytes against chemo- and radiation therapy by figuring out the key target molecules and effective inhibitors. There are several candidate inhibitors so far such as GnRH analogues (5), S1P (6), AS101 (7), and Imatinib (8). But there is still controversy as to how to protect oocytes against chemo- and radiation therapy. None of these have yet been tested in mice to kill cancer cells while protecting the ovarian reserve. If CHK2 is a key molecule in the signaling pathway of oocyte death as Schimenti’s team proposed, the use of CHK2 inhibitor could be an ideal way to protect oocytes against apoptotic pathway by radiation and give time to repair DNA damage in oocytes while killing cancer cells.
We cannot determine the healthiness of oocytes undergoing these treatments although the inhibitors could prevent the death of oocytes during chemo- and radiation therapy in cancer patients. Although the radiated oocytes from Chk2-/- mice produced healthy pups, the team would need to pursue much deeper research to study the genomes of the pups to determine if they contain DNA mutations when CHK2 inhibitors are used. While more research needs to be done, this is another step towards fertility preservation in cancer patients.
References:
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343(6170):533-536.
- Wang XQ, Redpath JL, Fan ST, Stanbridge EJ. ATR dependent activation of Chk2. Journal of cellular physiology. 2006;208(3):613-619.
- Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, De Stanchina E, Bristow RG, Suda T, Lowe SW, Jeggo PA, Elledge SJ, Mak TW. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Molecular and cellular biology. 2002;22(18):6521-6532.
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444(7119):624-628.
- Osborne SE, Detti L. GnRH-analogues for ovarian protection in childhood cancer patients: how adult hypotheses are relevant in prepubertal females. Current drug targets. 2013;14(8):856-863.
- Zelinski MB, Murphy MK, Lawson MS, Jurisicova A, Pau KY, Toscano NP, Jacob DS, Fanton JK, Casper RF, Dertinger SD, Tilly JL. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertility and sterility. 2011;95(4):1440-1445 e1441-1447.
- Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Science translational medicine. 2013;5(185):185ra162.
- Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, Mattei M, Candi E, De Felici M, Melino G, Cesareni G. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nature medicine. 2009;15(10):1179-1185.


 By: Brigid Martz Smith -
By: Brigid Martz Smith -