Klinefelter Syndrome
| Prevalence | Between 1 in 500 and 1 in 1000 males have Klinefelter syndrome [1]. Variants of Klinefelter syndrome occur more infrequently; approximately 1 in 50,000 or fewer newborns has a variant of Klinefelter syndrome[2].
Most men are diagnosed as adults in the context of male infertility. Only 10% of males with Klinefelter syndrome are diagnosed before age 14 years. Klinefelter syndrome is underdiagnosed because the condition is often not identified in men with mild signs and symptoms. Additionally, features can vary and overlap with other conditions[1]. Klinefelter syndrome is one of the leading causes of male infertility. Approximately 3% of all infertile men have Klinefelter syndrome[1] and 14% of non-obstructive azoospermic men have Klinefelter syndrome[3]. |
| Natural History | 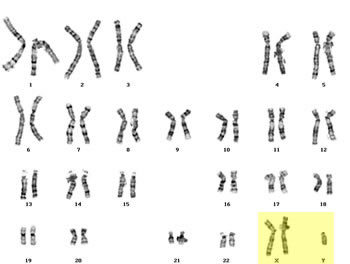 Klinefelter syndrome is a chromosomal condition that affects male physical and cognitive development. The syndrome is the result of one additional X chromosome, or a 47,XXY karyotype. The extra X chromosome interferes with male sexual development, often preventing the testes from functioning normally and reducing the levels of testosterone. Klinefelter syndrome is a chromosomal condition that affects male physical and cognitive development. The syndrome is the result of one additional X chromosome, or a 47,XXY karyotype. The extra X chromosome interferes with male sexual development, often preventing the testes from functioning normally and reducing the levels of testosterone.
Some individuals have a variant of Klinefelter syndrome- meaning they have more than one additional X chromosome, such as a 48,XXXY or 49,XXXXY karyotype. These individuals may experience more severe signs and symptoms. Klinefelter syndrome and its variants are not inherited. Klinefelter syndrome occurse due to a random event when the reproductive cells (eggs and sperm) are forming in the parent. The random event is called nondisjunction and the resulting reproductive cell has an abnormal number of chromosomes. Nondisjunction is often associated with increased maternal age[1]. The mosaic forms of Klinefelter syndrome, like 46,XY/47,XXY are also not inherited. Mosaic Klinefelter syndrome results from a random event in cell division early in fetal development. As a result- there are two cell lines within the body. Individuals with mosaic Klinefelter may have less severe signs and symptoms. |
| Disease Presentation | 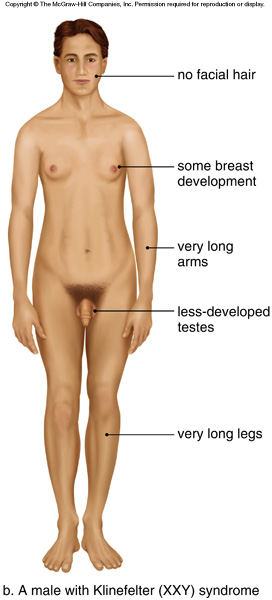 The signs and symptoms of individuals with Klinefelter syndrome (47,XXY) and its variants tend to vary among affected individuals[2]. The following are characteristic of Klinefelter syndrome: The signs and symptoms of individuals with Klinefelter syndrome (47,XXY) and its variants tend to vary among affected individuals[2]. The following are characteristic of Klinefelter syndrome:
There is also an increased risk for breast cancer and systemic lupus erythematosus. Individuals with variants of Klinefelter (more than one extra chromosome, like 48,XXXY and 49,XXXXY) may have intellectual disabilities, distinctive facial features, skeletal abnormalities, poor coordination, and severe speech problems. As the number of extra sex chromosomes increases, so does the risk of developing these more severe health problems[2]. |
| Effect on Fertility | Individuals with Klinefelter syndrome typically have microorchidism (small testes) and do not produce adequate levels of testosterone. Testosterone directs male sexual development before birth and during puberty. A shortage of testosterone can lead to delayed or incomplete puberty, gynecomastia, reduced facial and body hair, and infertility[2].
Infertilty is typically due to severe spermatogenesis impairment responsible for azoospermia in ~90% of men with Klinefelter syndrome (47,XXY) and ~75% of men with mosaic Klinefelter syndrome[4]. |
| Fertility Preservation Options | Fertility preservation may be best proposed to adolescent Klinefelter patients, just after the onset of puberty, when it is possible to collect a semen sample and when the patient is able to consider alternative options to achieve fatherhood and also accept the failure of spermatozoa or immature germ cell retrieval[5]. Additionally, successful sperm retrieval may decrease with age and after testosterone therapy, further warranting fertility preservation during adolesence[3]. The following are methods for preserving fertility in those with Klinefelter syndrome:
TESE invovles extracting viable sperm cells from testicular tissue after a testicular biopsy. Pening et al. (2014) reported successful recovery in 20% of study participants with Klinefelter syndrome who underwent TESE[7]. TESE can be combined with itracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF); it offers the opportunity for Klinefelter patients with azoospermia to father children with their own spermatozoa. However, successful retrieval decreases with age and after testosterone therapy[3], therefore it is important to discuss the option of TESE with adolescents or quickly after diagnosis of Klinefelter syndrome in adult men. Normal fertilization, embryo development, pregnancies, and births have been achieved after TESE and ICSI for men with Klinefelter syndrome[6]. Spermatogonial Stem Cell (SSC) Banking Germ cell depletion begins at the onset of puberty and leads to infertility, therefore banking SSCs, also called immature germ cells, has been proposed as a strategy to preserve fertility in adolescents with Klinefelters syndrome. For optimal preservation of SSCs, spermatogonia should be retrieved by testicular biopsy preferably before the testis hyalinization occurs[8]. Sperm Banking For those without azoospermia or before undergoing testosterone replacement therapy, sperm cells may be collected after ejaculation and frozen for future use. This may be a better option for those men with mosaic Klinefelter syndrome, as most men with Klinefelter have azoospermia. Read more information about sperm banking. Couples considering having biological children should be offered the option of preimplantation genetic diagnosis (PGD) to ensure the embryo selected in karyotypically normal[3]. |
References
[1]Bojesen, A., Juul, S., & Gravholt, C. (2003). Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab, 88, 622-626.
[2]Klinefelter syndrome. (2013). U.S. National Library of Medicine.http://ghr.nlm.nih.gov/condition/klinefelter-syndrome Retrieved May 20, 2014
[3]Krausz, C., & Chianese, C. (2014). Genetic testing and counseling for male infertility. Curr Opin Endocrinol Diabetes Obes, 21(3), 244-250.
[4]Mau-Holzmann, U. (2005). Somatic chromosomal abnormalities in infertile men and women. Cytogenet Genome Res, 111, 317-336.
[5]Rives, N., Milazzo, J., Perdrix, A., Castanet, M., Joly-Helas, G., Sibert, L., . . . Mace, B. (2013). The feasibility of fertility preservation in adolescents with Klinefelter syndrome. Hum. Reprod., 28(6), 1468-1479.
[6]Fullerton, G., Hamilton, M., & Maheshwari, A. (2010). Should non-mosaic Klinefelter syndrome men be labeled as infertile in 2009? Hum Reprod, 25(588-597).
[7]Pening, D., Delbaere, A., & Devreker, F. (2014). Predictive factors of sperm recovery after testicular biopsy among non-obstructive azoospermic patients. Obstet. Gynecol., 123(Suppl 1), 189S-190S.
[8]van Saen, D., Gies, I., & De Schepper, J. (2012). Can pubertal boys with klinefelter syndrome benefit from spermatogonial stem cell banking? Hum Reprod, 27, 323-330.
About the Author
Allison Goetsch, MS, CGC is a pediatric genetic counselor at Ann and Robert H. Lurie Children’s Hospital of Chicago and a member of the Oncofertility Consortium administrative core team. She completed her graduate thesis with Dr. Teresa K. Woodruff researching oncofertility and hereditary breast and ovarian cancer (HBOC) syndrome. Allison’s primary goals are to increase fertility preservation awareness and education for both health care providers and patients regarding malignant and non-malignant diseases (and/or treatments) which threaten fertility.
This page was last updated February 6th, 2016.


 Testicular Sperm Extraction (TESE)
Testicular Sperm Extraction (TESE)