Turner Syndrome
Turner syndrome (TS) is the most common sex chromosome disorder with an incidence of 1/2500 live-born females. This condition occurs with a similar frequency in all populations.
Natural History
Turner syndrome is a developmental disorder and is caused by the absence or structural abnormality of a sex chromosome, typically an X chromosome. This syndrome is an instance of monosomy, and it is the only one that is not lethal once the child is born. The karyotype of a girl with classic Turner syndrome is typically (45, X) (instead of 46, XX or 46, XY). However, some girls with Turner syndrome have what is called mosaicism; not all cells are missing the second X chromosome. Their karyotype is (46,XX/45,X).
Disease Presentation
(Seen in >50% girls): short stature, low bone mineral density, learning disabilities, and delayed puberty from early ovarian failure. In particular, Turner syndrome most noticeably affects growth and endocrine health. Pediatric endocrinologists have established practice guidelines for management of Turner syndrome, including thyroid function tests starting at age four, and evaluation for growth and pubertal development starting at age 10. For issues with growth, girls receive growth hormone therapy (GHT) injections. Without GHT, the average height of a girl with TS is <5%ile by age two. Thus, GHT is considered standard of care once growth failure is demonstrated, and can help improve final adult height. Because of ovarian failure, many girls are not able to enter puberty on their own. Estrogen replacement therapy (ERT) is often used starting at around age 12 years for girls with TS who have not yet gone through spontaneous puberty. This has been successful in promoting pubertal development (Gonzalez, 2012). Although ERT helps commence puberty, most girls with Turner syndrome are infertile due to ovarian failure. Regardless of karyotype, 95% of girls with TS develop ovarian failure over time. In girls with TS, ovarian failure may start as early as 18 weeks into fetal life. However, not every girl has complete ovarian failure at birth. The timeline and rate at which this occurs is less clear, and may be different for each woman with Turner syndrome. Viable oocytes have been found in some girls with classical TS (45, X) and 1-2% of women with TS have spontaneous pregnancies. So, while ovarian failure is a hallmark of Turner syndrome, not every girl will face infertility, and there may be time before ovarian failure occurs to preserve fertility.
Why Ovarian Failure?
The prevailing hypothesis for ovarian failure is the faulty meiotic pairing in cell division of a 45,X germ cell that leads to accelerated apoptosis of germ cells. Studies confirm that viable oocytes arise from 46,XX germ cells and 45,X germ cells are eliminated. Thus, individuals with 46,XX/45,X mosaicism in their germ line theoretically have more viable oocytes than those without mosaicism. This is predicted to have direct implications on ovarian failure and thus possible fertility preservation options. While the genetic mechanism of ovarian failure has been studied, the timeline and rate at which this occurs is less clear, and may be different for each woman with Turner syndrome.
Current Reproductive Options for Women with Turner syndrome
Women with Turner syndrome currently have limited reproductive options. In 2% of women, spontaneous pregnancy can occur. However, if the woman has heart complications, pregnancy can be dangerous. Aortic dissection, which occurs in 10% of TS pregnancies, causes maternal death in 2% of pregnant women with TS. Thus, for patients with heart problems, pregnancy is not recommended. For those who become pregnant, doctors recommend surveillance for hypertension and periodic echocardiograms throughout the pregnancy. If the woman wants to carry her own pregnancy, there have been reports of successful in vitro fertilization (IVF) (single embryo transfer) pregnancies through oocyte donation, often donated from the woman’s mother. While preserving biological relatedness, this can be a socially uncomfortable option for these women. Alternatively, adoption is an option for women with Turner syndrome who are infertile or do not want to risk pregnancy or cannot afford gestational surrogacy or oocyte donation.
Fertility Preservation
Oncofertility® is a multidisciplinary field that incorporates oncology and reproductive endocrinology and aims to provide future reproductive options for cancer survivors. Cancer treatments including chemotherapy and radiation can often result in infertility for women. Cryopreservation of ovarian follicles for future use before undergoing cancer treatment creates the possibility for women to have biological children after surviving cancer. With time as an important factor in successfully treating cancer, ovarian cryopreservation is often preferred over ovarian stimulation, which takes a few weeks to complete. The process of cryopreservation involves laparoscopic surgery to remove ovarian tissue containing immature oocytes. The tissue is then frozen in strips, to be matured and used later by the patient, either through in-vitro fertilization or transplantation (Figure 1). This process has been largely successful for cancer patients.
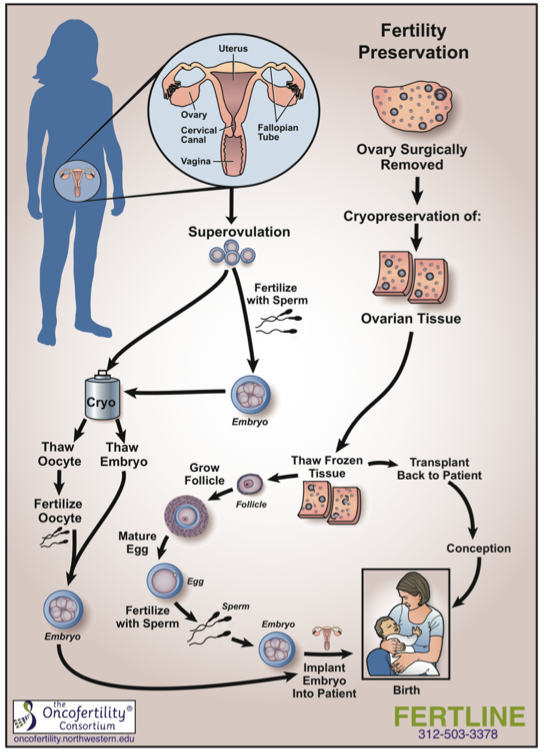
Figure 1. Fertility Preservation Process
The benefits of follicle retrieval and cryopreservation can reach beyond cancer patients as the process theoretically applies to other women facing infertility. With current research in preserving fertility in patients with Turner syndrome, expansion of the Oncofertility® model to this population is seemingly inevitable. However, many questions remain and must be addressed. Some of these considerations include determining the best time to take ovarian follicles; which girls would benefit from fertility preservation; the yield of viable oocytes from the woman; and whether surrogacy is a viable option when pregnancy is contraindicated due to cardiovascular risks.
Research on Fertility Preservation in Turner syndrome
Mosaicism in TS germ cells tends to lessen the degree of ovarian failure (Visser et al., 2013). However, it can be difficult to know the extent of mosaicism present in the germline, as a blood karyotype does not indicate mosaicism in germ cells. In an effort to address the issue of gonadal dysgenesis in Turner syndrome, researchers have discovered that Anti-Müllerian hormone (AMH) is an indicator of ovarian reserve and function (Borgstrom et al., 2009, Hagan et al., 2010). Girls with mosaic karyotypes more often had measurable levels of AMH, indicating that they may have viable eggs. Just 10% of girls with 45,X karyotypes had AMH levels that would suggest fertility (Visser et al., 2013). This research has shown that the level of mosaicism is correlated with positive fertility indicators, but further studies on ovarian failure are needed to more precisely pinpoint the age at which fertility can be best preserved.
Recently, oocyte cryopreservation has been pursued in research as a possible reproductive option for women with Turner syndrome. Case reports provide some evidence that patients with mosaic karyotypes tend to have more potentially viable eggs than those with a 45,X karyotype. A study by Oktay et al. (2010) described a 14 year old with mosaic Turner syndrome who underwent two cycles of controlled ovarian stimulation and oocyte cryopreservation. Eleven oocytes were retrieved and cryopreserved by vitrification. The girl has not yet used the frozen oocytes to attempt a pregnancy, but the process of retrieval was successful. Another recent case study involved a 16 year old with mosaic Turner syndrome who underwent a successful laparoscopic wedge resection. Eight oocytes that matured were cryopreserved by vitrification (Huang et al., 2008). Similar to the first case, the woman has not yet tried to achieve a pregnancy with these retrieved oocytes. It is important to note that while oocyte cryopreservation has been successful in case reports, because this process is very new, successful pregnancy has not yet been reported (Hewitt et al., 2013).
Moving Forward
It is known that most girls with Turner syndrome have complete ovarian failure by the end of puberty. The best time to try fertility preservation is unknown. While failure can begin as a fetus, it is believed that younger girls are most likely to have more viable oocytes than their older counterparts. This likely means surgical intervention for preservation before the child can give consent; this must be addressed with patients and parents. The science behind preservation for girls with Turner syndrome is yet to be perfected, but it is a promising approach to providing fertility options to the Turner syndrome population in the near future.
References:
Borgstrom, B., Hreinsson, J., Rasmussen, C., Sheikhi, M., Fried, G., Keros, V., Hovatta, O. (2009). Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab, 94(1), 74-80.
Hagen, C. P., Aksglaede, L., Sorensen, K., Main, K. M., Boas, M., Cleemann, L., Juul, A. (2010). Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab, 95(11), 5003-5010.
Hewitt, J. K., Jayasinghe, Y., Amor, D. J., Gillam, L. H., Warne, G. L., Grover, S., & Zacharin, M. R. (2013). Fertility in Turner syndrome. Clin Endocrinol (Oxf), 79(5), 606-614.
Huang, J. Y., Tulandi, T., Holzer, H., Lau, N. M., Macdonald, S., Tan, S. L., & Chian, R. C. (2008). Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: Case Report. Hum Reprod, 23(2), 336-339. doi: 10.1093/humrep/dem307
Oktay, K., Rodriguez-Wallberg, K. A., & Sahin, G. (2010). Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril, 94(2), 753.e715-759. Visser, J. A., Hokken-Koelega, A. C., Zandwijken, G. R., Limacher, A., Ranke, M. B., & Fluck, C. E. (2013). Anti-Mullerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum Reprod, 28(7), 1899-1907.

