Site: Northwestern University
Principal Investigator: Lonnie D. Shea, PhD
Rationale
 For folliculogenesis to properly occur, it is essential to maintain the intimate physiological connections between the oocyte and surrounding somatic cells. To recapitulate the 3-dimensionality of the ovary and maintain the appropriate size, shape, and architecture of the ovarian follicle while providing the necessary stimuli to direct cellular responses, a synthetic scaffold must serve as an in vitro mimic of the in vivo ovarian microenvironment.
For folliculogenesis to properly occur, it is essential to maintain the intimate physiological connections between the oocyte and surrounding somatic cells. To recapitulate the 3-dimensionality of the ovary and maintain the appropriate size, shape, and architecture of the ovarian follicle while providing the necessary stimuli to direct cellular responses, a synthetic scaffold must serve as an in vitro mimic of the in vivo ovarian microenvironment.
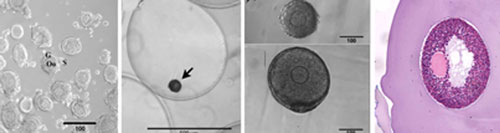
Previous work has demonstrated that the hydrogel alginate phenocopies the in vivo ovarian environment by maintaining follicular architecture and permitting combinations of diffusible, insoluble, and mechanical signals to influence the development of the follicle. Using this scaffold, murine ovarian follicles have been successfully matured in vitro to yield high-quality oocytes that were fertilized and used for the birth of live, viable offspring.
The application of biomaterials and tissue engineering to reproductive biology provides an enabling technology that is central to the Oncofertility Consortium®. The work of the biomaterials core directly supports the work of other Consortium members; in particular, growth of large nonhuman primate and human follicles that may have different requirements from mouse follicles, maintaining follicle architecture and facilitating handling during cryopreservation, and promoting engraftment and survival of primate follicles following autotransplantation of ovarian tissue.
The objectives of this project are to:
- Provide biomaterial support to the Oncofertility Consortium®
- Identify biomaterial properties and culture conditions to maximize primate follicle growth and transfer knowledge to appropriate projects
- Develop novel biomaterials that can be used to minimize tissue cryoinjury
- Engineer drug-releasing hydrogels to optimize ovarian cortical strip transplants
Key Experiments
- Assess the mechanical influence of the ovarian stroma on follicle development using the alginate culture system
- Perform thermal analysis and polymer molecular weight measurements before and after follicle cryopreservation to identify optimal alginate properties and cooling procedures for maximum survival of individual follicles and cortical strips
- Subcutaneously implant alginate hydrogels with immobilized ECM and angiogenic factors into the abdomen of mice and analyze cellular infiltration, identify cell types, and quantify blood vessel growth (vascularization) to gauge host tissue engraftment
Consortium Support and Impact
The Biomaterials Core provides critical service, support, and training to the other Oncofertility Consortium® projects. The exchange of biomaterials, development of techniques, training, and constant communication are central to the Core’s mission. In addition, there is reciprocal interaction as results from the individual projects feed into the Core to further optimize 3-D culture systems.
Publications
Shikanov A, Smoith R M., Xu M, Woodruff T K., Shea L D. Hydrogel Network Design Using Multifunctional Macromers to Coordinate Tissue Maturation in Ovarian Follicle Culture. Biomaterials. 2011. April; 32(10): 2524-31.
Silber S J., Woodruff T K., Shea D L. To Transplant or Not to Transplant – That is the Question. Cancer Treatment and Research. 2010; 156: 41-54. PMID: 20811824.
Smith, R M., Woodruff T K., Shea L D. Designing Follicle-Environment Interactions with Biomaterials. Cancer Treatment and Research. 2010; 156: 11-24. PMID 20811822.
Weiss MS, Peñalver Bernabé B, Bellis AD, Broadbelt LJ, Jeruss JS, Shea LD. Dynamic, large-scale profiling of transcription factor activity from live cells in 3D culture. PLoS One. 2010 Nov 17;5(11):e14026.PMID: 21103341
Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating Fibrin–Alginate Matrices For In Vitro Ovarian Follicle Development. Biomaterials Vol. 29 5476-85 Oct 30, 2009
West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, and Shea LD. The Mouse Follicle Microenvironment Regulates Antrum Formation and Steroid Production: Alterations in Gene Expression Profiles. Biology of Reproduction, 80, 432–439, 2009.
Xu M, Banc A, Woodruff TK, Shea LD. Secondary Follicle Growth and Oocyte Maturation by Culture in Alginate Hydrogel Following Cryopreservation of the Ovary or Individual Follicles. Biotechnology and Bioengineering, Vol. 103, No. 2, June, 2009.
West-Farrell ER, Xu M, Woodruff TK, Shea LD. Physical Properties of Alginate Hydrogels and Their Effects On In Vitro Follicle Development. Biomaterials Vol 30 4439-48 Oct 2007.
Woodruff TK and Shea LD. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod Sci. Vol 14 No 8 Suppl 6-10 Dec 2007
Xu M, PhD, Woodruff TK, and Shea LD. Bioengineering and the Ovarian Follicle. Cancer Treat Res. 2007;138:75-82.
Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of Extracellular Matrix Proteins Type I Collagen, Type IV Collagen, Fibronectin, and Laminin In Mouse Folliculogenesis. Histochem Cell Biol Vol 126 No 5 583-92 Nov 2006.
Kreeger PK, Deck JW, Woodruff TK, and Shea LD. The In Vitro Regulation Of Ovarian Follicle Development Using Alginate-Extracellular Matrix Gels. Biomaterials Vol 27 No 5 714-23 2006.
Kreeger PK, Fernandes NN, Woodruff TK, and Shea LD. Regulation of Mouse Follicle Development by Follicle-Stimulating Hormone in a Three-Dimensional In Vitro Culture System Is Dependent on Follicle Stage and Dose. Biology of Reproduction Vol 73 No 5 942-50 June 29 2005.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and PL1EB008542.

