Aplastic Anemia
| Incidence | Aplastic anemia (AA) occurs in two to four subjects per million population per year, according to Marsch (2009). |
| Natural History | Aplastic anemia is a disease in which all three cell lines (red blood cells, white blood cells, and platelets) in the bone marrow are destroyed or absent. Thus, there is a deficiency in all three mature cell type (so the name “anemia” is a misnomer, as it is in fact a “pancytopenia). The term “aplastic” means that the bone marrow cannot make these mature cells from stem cells. It is most common in the teenage years in both males and females.
Treatment Aglobulin (ATG) is a horse or rabbit-derived antibody to fight T cells)). Cyclosporine is used to suppress the immune system. One of the newer treatments is eltrombopag, which was approved in February 2014. More severe cases and in younger patients who have an HLA matched sibling, bone marrow transplant (BMT) can be curative. The stem cells in the donor’s bone marrow are able to produce all three cell lines. |
| Disease Presentation | Patients usually present with symptoms related to the low blood counts of the three cell lines. For example,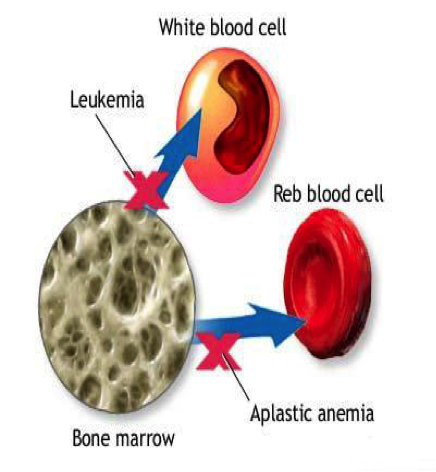
Low Hemoglobin / Red Blood Cells (Anemia):
Low White Blood Cells (Leukopenia)
Low Platelets (Thrombocytopenia)
Testing to Confirm Diagnosis, according to Marsch (2009)
Image: Bone marrow filled with adipocytes (fat cells) instead of WBC, RBC, and platelets |
| Effect on Fertility | 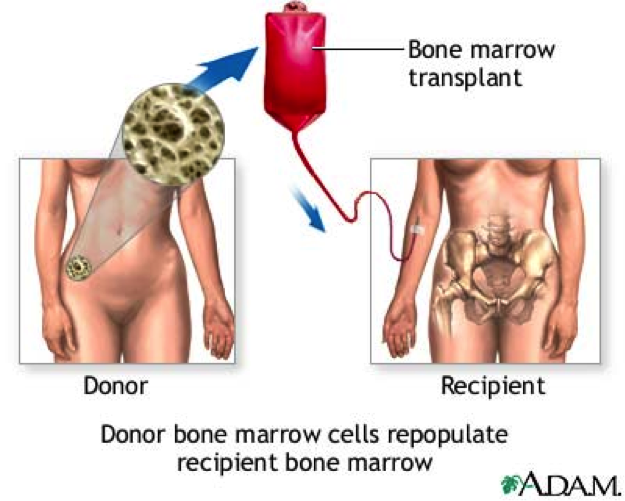 The effects on fertility for patients with AA depend on the type of treatment received (ie: chemotherapy and/or stem cell bone marrow transplant). According to Marsh (2006), AA may occur with pregnancy and sometimes resolves spontaneously after delivery or termination of pregnancy. There is no evidence of reduced fertility in acquired AA after treatment with ATG or cyclosporine. However, AA may relapse in more than one third of patients when they become pregnant again. There are no increased birth defects or spontaneous abortions in mothers taking cyclosporine, so this is the main treatment during pregnancy. The effects on fertility for patients with AA depend on the type of treatment received (ie: chemotherapy and/or stem cell bone marrow transplant). According to Marsh (2006), AA may occur with pregnancy and sometimes resolves spontaneously after delivery or termination of pregnancy. There is no evidence of reduced fertility in acquired AA after treatment with ATG or cyclosporine. However, AA may relapse in more than one third of patients when they become pregnant again. There are no increased birth defects or spontaneous abortions in mothers taking cyclosporine, so this is the main treatment during pregnancy.
Currently, there are no birth defects or infertility issues associated with taking ATG and cyclosporine. With these two drugs and an HLA matched sibling bone marrow transplant, they can preserve fertility. With that said, this has only been the treatment regimen for between five and ten years, it cannot be said with certainty that there are no infertility problems. However, with other treatment regimens and non-HLA matched donors, there may be infertility risks. According to Haberman in his 1991 study, of the nine male renal transplant patients treated with cyclosporine, three of the four were able to have a child with their spouses. Testicular hormone function and semen analysis was normal in eight of the nine patients. However, because many of these patients require stem cell BMT, the effects on fertility must be discussed with this as well. According to Tauchmanovà in his 2005 study on autologous bone marrow transplant and its effect on the endocrine system, 93% of women had precocious ovarian failure and 37% of men showed low testosterone levels after autologous stem cell transplant. Even after one year, semen analysis showed decreased sperm count in 91% of the males. Thus, with these decreased levels of hormones, fertility decreased in both males and females. Spermatogenesis and egg viability may also be a product of patient age and other associated factors while undergoing allogeneic hematopoietic stem cell transplant. According to Rovoa (2006), nine of the sixteen patients undergoing transplantation who were under twenty five did show some degree of spermatogenesis.
|
| Fertility Preservation | Since both males and females are at risk of infertility following stem cell transplant, there are several fertility options for these individuals that should be discussed before treatment / transplant.
According to Loren (2013), these patients at risk should undergo pretreatment counseling with individualization of fertility preservation depending on necessary forseen treatment, patient age, time available, costs, and long term issues. Cryopreservation of embryos, oocytes, ovarian tissue, semen, and testicular tissue are proven effective techniques for preserving reproductive function.
For males with aplastic anemia, cryopreservation of ejaculated sperm is well established technique and should be offered to all post-pubertal males. If the patient is unable to ejaculate or no sperm are found, they may recover sperm by epididymal sperm aspiration or testicular sperm extraction, as noted by Chan PT (2001). |
References
- Marsh, J. “Making Therapeutic Decisions in Adults with Aplastic Anemia.” American Society of Hematology Education Book. January 1, 2006. Vol 2006. No 1:78-85
- Haberman, J., Karwa, G., et al. “Male fertility in cyclosporine-treated renal transplant patients.” Journal of Urology. 1991 Feb;145 (2):294-6
- Tauchmanovà L, Selleri C, De Rosa G, Esposito M, Di Somma C, Orio F, Palomba S, LombardiG, Rotoli B, Colao A. “Endocrine disorders during the first year of autologous stem cell transplant.” Am J Med. 2005;118(6):664
- Rovoa, T., Passweg J., Heim D., Meyer-Monard, S., Holzgreve W. “Spermatogenesis in long term survivors after allogenic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GVHD.” Blood. 2006. 108(3):1100.
- Chan P., Palermo, G., Veeck., “Testicular sperm extraction combined with intracytoplasmic sperm injection in the treatment of men with persistent azoospermia postchemotherapy.” Cancer. 2001; 92-1632
- Loren, A., Mangu, P., Beck, L., Brennan, L. “Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update.” Journal of Clinical Oncology. 2013 July; 31(19):2500-10
- Oktay, K., Cil, A., Bang, H. “Efficiency of oocyte cryopreservation: a meta-analysis.” Fertility Sterility. 2006; 86(1):70.
- Marsh, J., Ball, S., Cavenagh, J., et al. “Guidelines for the diagnosis and management of aplastic anaemia.” British Journal of Haematology. 2009; 147:43.
About the Author
Kristen Wendell, DO is completing her residency in internal medicine at Advocate Lutheran General Hospital in Park Ridge, IL. She received her undergraduate degree from the University of Illinois in Champaign/Urbana (2007) and her medical degree from Midwestern University / Chicago College of Osteopathic Medicine (2013). As a healthcare provider with plans to complete a fellowship in hematology/oncology, she is working with the Oncofertility Consortium® team in order to provide awareness to other physicians regarding fertility preservation for patients with both malignant and non-malignant conditions.
Last Updated On: August 19th, 2014


 Causes
Causes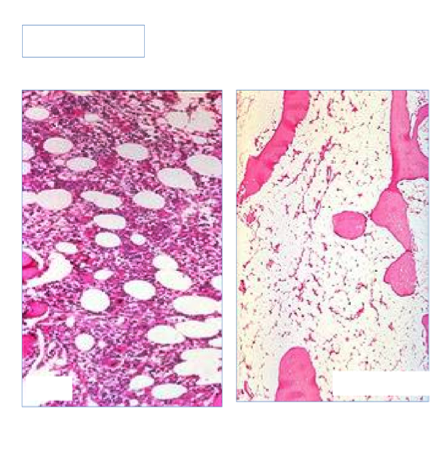
 Since gonadotoxic treatment is usually implemented soon after diagnosis, one should seek immediate counseling as the process for ovarian stimulation and oocyte retrieval can take two to three weeks.
Since gonadotoxic treatment is usually implemented soon after diagnosis, one should seek immediate counseling as the process for ovarian stimulation and oocyte retrieval can take two to three weeks.