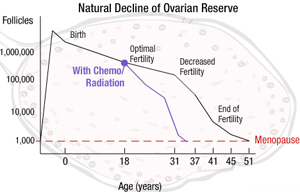
Assessing Ovarian Reserve After Cancer Treatments
Attempting to assess ovarian function after cancer treatments can be very challenging. Factors to consider include:
- Age at the time of chemotherapy and/or radiation
- Cumulative dose of chemotherapy and/or radiation
- Specific chemotherapeutic agents used
- Current age
- History of infertility
- Currently menstrual patterns, and/or other symptoms, such as vasomotor symptoms
| What does the literature tell us? | Many prior studies have looked at this issue. However, many of these studies were retrospective, and most specifically address menstrual cycles, not fecundity. To date, there is no prospective data specifically addressing likelihood of infertility after cancer treatments.
Summary data – risk of amenorrhea Lo Presti et al. summarizes the literature about various common chemotherapeutic regimens and the risk of amenorrhea1. Walshe et al. summarizes the data specifically for women with breast cancer2. Chemotherapy for breast cancer: One prospective, multi-center, observational study followed 466 women with breast cancer, using ‘bleeding calendars’3, 4. They find that:
|
| Childhood Cancer Survivor Study | The Childhood Cancer Survivor study follows survivors of childhood cancers, comparing reproductive outcomes to their siblings5. Some of their findings include:
|
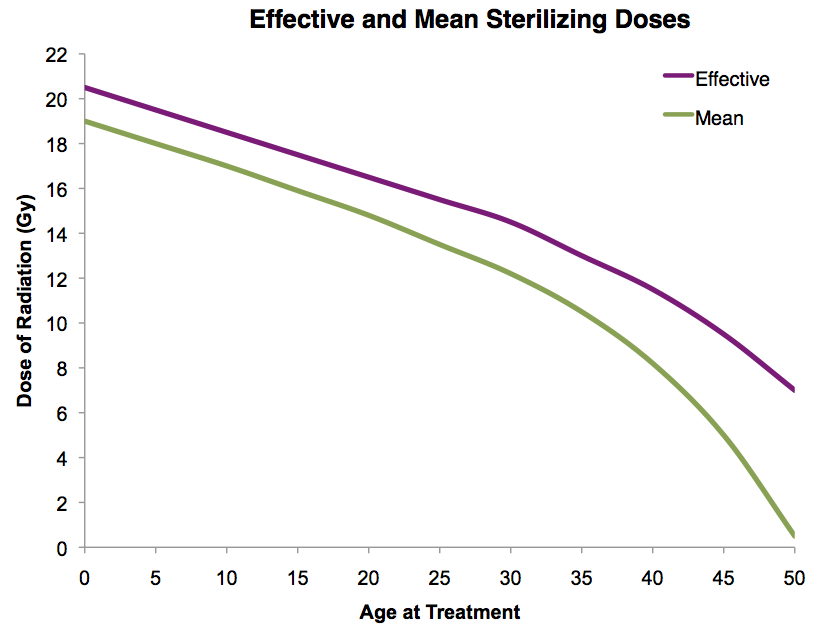
| Models to predict premature menopause? | Prior Radiation Therapy
Adapted from Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005;62:738-44.Prior Chemotherapy
|
| What does the presence or absence of menses indicate? | If the patient is NOT having menstrual cycles, what does this mean?
If the patient IS having menstrual cycles, is she fertile?
|
References
1. Lo Presti A, Ruvolo G, Gancitano RA, Cittadini E. Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol 2004;113 Suppl 1:S33-40.
2. Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 2006;24:5769-79.
3. Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol 2006;24:1045-51.
4. Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer 2010;116:3102-11.
5. Green DM, Sklar CA, Boice JD, Jr., Mulvihill JJ, Whitton JA, Stovall M et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2374-81.
6. Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005;62:738-44.
About the Author
Jennifer Mersereau, MD, MSCI, is an reproductive endocrinologist in the University of North Carolina’s Department of Obstetrics and Gynecology. As the Director of the Fertility Preservation Program, she has extensive experience guiding patients and physicians through the oncofertility experience.
This page was last updated March 14, 2012.