Testicular Tissue Cryopreservation
Testicular tissue cryopreservation is an option for prepubertal patients who are not producing sperm and therefore are unable to preserve a semen sample. It is important to emphasize that the methods described here are experimental, and that only a limited number of patients have cryopreserved their testicular tissue. No spermatogenic recovery or pregnancies from cryopreserved testicular tissues have been reported to date. However, there are several methods in the research pipeline suggesting that preserved testicular tissue or cells can be used to restore fertility and/or produce sperm. Anticipating that new therapies will be available in the future, many centers in the US and abroad have determined that it is reasonable to preserve testicular tissue for young patients who are at high risk for infertility and have no other options to preserve their fertility.
Experimental options that may be available in the future for patients who preserve testicular tissue are transplantation of spermatogonial stem cells (SSC) back into the testes of the patient; maturation of spermatozoa in testicular tissue organ culture and testicular tissue autografting or xenografting. The following paragraphs will describe, in brief, the current status of these research areas.
It is important to note that except for SSC transplantation, all of these methods will require the use of ICSI to achieve fertilization and pregnancy. ART, including IVF with ICSI is associated with additional financial, physical, and psychological risks and should be discussed with the patient families early on.
Spermatogonial Stem Cell Transplantation
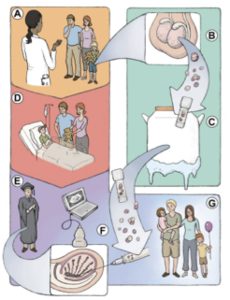
Testicular tissue contains spermatogonial stem cells (SSCs) that can be isolated from frozen tissue pieces and transplanted back into the seminiferous tubules of the patient’s testicles to produce sperm. This is the only method that has the potential to restore normal spermatogenesis and fertility in survivors (Fig. 1). Spermatogonial stem cell transplantation was first established in mice [7,8] and has since been successfully performed in other animals including non-human primates [9,10]. This approach has been attempted in a few men but the outcomes of those transplants have not been reported [11].
Fig.1. Fertility preservation in prepubertal cancer patients (adopted from Hermann and Orwig (Eds) “Male Germline Stem Cells: Developmental and Regenerative Potential”, Springer 2011.)
Tissue Grafting
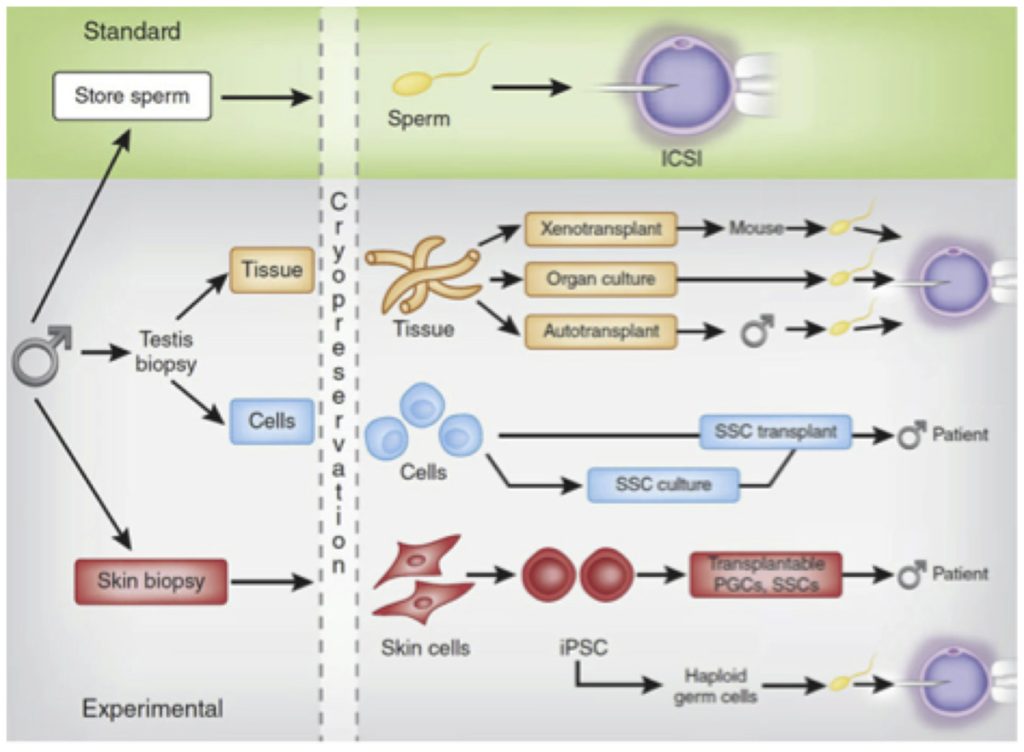
An alternative to transplanting disaggregated testis cells (including SSCs) back into the seminiferous tubules of the testes is to graft intact testicular tissue pieces to an orthotopic (into the scrotum) or an ectopic site (e.g. under the skin) to initiate spermatogenesis from SSCs contained in the tissue. Sperm can be recovered from the tissue after several weeks and used to achieve fertilization by ICSI (Fig. 2). Production of spermatogenesis and sperm from autografted testicular tissues has been reported in mice and non-human primates [12,13]. In humans, autologous testicular tissue grafting has not been attempted yet.
Autologous SSC transplantation or testicular tissue grafting should not be considered in cases where malignant contamination in the tissue is suspected. In these cases, inter-species xenografts (e.g. from human to mouse or human to pig) might be used to generate sperm for IVF-ICSI (Fig. 2). The production of fertilization-competent sperm in testis tissue grafted to immunosuppressed mice has been reported for non-human primates [14], but production of sperm has not yet been achieved with human testicular tissue xenografts [15-20].
FUTURE DIRECTIONS
In vitro SSC culture
SSC in vitro culture systems are being developed to expand SSCs from small tissue biopsies (Fig. 2). This approach has the potential to generate millions of stem cells for injection into the testis, increasing the chances for robust engraftment and initiation of spermatogenesis. Methods for culturing rodent SSCs are well established where the cultured cells are competent to regenerate spermatogenesis and produce viable offspring [21-23]. Several groups have reported maintaining non-human primate [24] and human SSCs in culture [25-34] but none of these reports have been replicated in other laboratories and transplantation to demonstrate spermatogenic potential and fertility is not possible in the human system. Methods to assess the function of human cells are needed to validate culture methods before they are translated to the clinic.
In vitro spermatogenesis
If frozen tissue or cells obtained from frozen tissues could be cultured in vitro to form sperm this would eliminate the need for SSC transplantation or autografting, and would circumvent the risk of introducing malignant cells back into the patient. Alternative methods have been explored in mice [35-37], and life pups were born after ICSI using haploid round spermatids and sperm matured in organ cultures of newborn mouse testicular tissue pieces [36-37]. This method has not been reported for large animals including primates.
Pluripotent-derived male germ cells
For infertile individuals who did not preserve germ cells before gonadotoxic therapy, induced pluripotent stem cells (iPSCs) may be produced from their somatic cells (e.g., skin or blood). In mice, iPSCs can be differentiated into transplantable germ cells that regenerate spermatogenesis and produce viable offspring [38]. These results have not been replicated in other species and transplant studies with human cells are not possible. However, several studies have reported that germ cells can be derived from human embryonic stem cells [39-42] and iPS cells [39,43] in vitro and have suggested that haploid cells can be generated, albeit at very low efficiency. Significant progress in germ cell derivation, expansion, and safety has to be made before this technology will be applicable clinically. In theory, this technology could allow men who did not preserve semen or testicular tissue to father biological children using sperm generated from their somatic cells.
Fig.2. Standard and experimental options for preserving male fertility (adopted from Valli et al. Fertil Steril2014;101:3–13. [44])
TTC is generally not recommended for patients who:
- Have entered puberty and have started spermatogenesis and can be referred to sperm banking.
- Have received prior chemotherapy that has significantly impaired testicular function.
Special Circumstances with TTC
Klinefelter Syndrome (KS)
KS is characterized by severe oligo- and azoospermia. In fact, 90% of KS cases are diagnosed in the context of fertility workup. For azoospermic KS patients, TESE has been used to recover sperm for ICSI with sperm retrieval and pregnancy rates similar to those reported for non-KS non-obstructive azoospermic patients [45,46].
A few centers are cryopreserving testicular tissue biopsies or sperm retrieved by TESE for adolescent Klinefelter patients [47-50]. However, the need for early intervention in this patient population has been debated because of the success of TESE-ICSI in adult KS patients [51].
Klinefelter mosaics (47,XXY/46,XY) are generally presenting with a milder phenotype and are more often oligospermic than non-mosaic patients [52], and the probability of recovering sperm by TESE may be greater for mosaic KS patients [48].
The cause and the course of spermatogenesis cessation in KS during puberty remains unknown and significant research will have to be done to better understand spermatogenic decline in KS, and to develop adequate fertility preservation strategies for this unique patient population
Table 1: Progress in Spermatogonial Stem Cell technology
| Rodents | Domestic and companion animals | Non-human Primate | Human | |
|---|---|---|---|---|
| SSC transplant | Progeny | Progeny | Fertilization & preimplantation embryo development | Attempted, result not reported |
| SSC culture | Progeny | Long-term survival of bovine and porcine spermatogonia | Short-term culture | Short-term survival and proliferation in vitro |
| Autografting of testicular tissue | Progeny (homologous graft) | Not attempted | Spermatogenesis | Not reported |
| Xenografting of testicular tissue | Not attempted | Spermatogenesis | Fertilization competent sperm | Long-term survival and presence of spermatocytes in prepubertal /adolescent tissue grafts. Limited survival of adult tissue grafts. |
| In vitro maturation of germ cells in organ culture | Progeny | Not reported | Not reported | Not reported |
| Generation of germ cells from iPS cells | Progeny | Future direction (not achieved) | Derivation of germ cells confirmed by biomarker expression | Derivation of germ cells confirmed by biomarker expression |

