Research Projects
The Oncofertility Consortium and its Center for Reproductive Health After Disease have current and past research programs that address the reproductive health needs of cancer and disease patients. To meet its goals, the team of researchers involved with the Oncofertility Consortium has undertaken various projects that reach across several diverse disciplines, with the ultimate goal of exploring and expanding options for the reproductive and fertility futures of patients.
A Shared Decision-Making Model: Informed Choice and Oncofertility Treatments
Site: Northwestern University
Principal Investigators: Paul Arntson, PhD; Marla Clayman, MPH, PhD; and Kathleen Galvin, PhD
Overview
This project explores how families facing a child’s cancer diagnosis decide whether or not to pursue fertility preservation treatments and the role that others, such as health care practitioners and religious counselors, play in this decision. The overall aim of this research is to gain a nuanced understanding of how families facing a child’s medical crisis communicate to make health care decisions and to develop a shared decision-making model that will facilitate informed choice in the clinical setting.
Objectives
- Understand how families, patients, and health care providers make “informed choices” about fertility preservation options when a young woman has been diagnosed with cancer.
- Construct a functional shared decision-making model for families, patients, and health care providers to use in making informed choices about fertility issues for young women diagnosed with cancer.
- Develop, utilize, and evaluate multiple formats of a shared decision-making model for families, patients, and health care providers that can be used in the clinical setting to help them make informed choices about fertility preservation options before cancer treatment.
Indices of Scholarship
- Conduct in-depth interviews with parents of young women and girls diagnosed with cancer when they were a minor to learn about what they knew regarding the potential effects of cancer treatment on fertility and how they arrived at decisions regarding fertility preservation options. These interviews will also explore who they turned to for advice and information, how they determined what they needed to know in order to make a decision, how their values were incorporated into a decision, and how they weighted information received in arriving at a decision. Depending on the age of the patient and parental consent, interviews will also be conducted with daughters.
- Conduct interviews with a representative group of health care providers to assess how they share information regarding cancer and fertility that aid in shaping the decision-making process
- Develop and pilot test a model of shared decision-making where informed choice is the goal. Once the model has been tested, design multiple formats (print, CD-ROM, interactive Web site) of this model to be used as decision aids in family education programs and as practical aids in the clinical setting.
Consortium Support and Impact
This project draws on other parts of the Oncofertility Consortium in developing a social science inquiry that is informed by scientific innovation and insight. It relies on clinical and medical perspectives and advances and application of oncofertility technology (see Follice Cryopreservation, Bioengineering Primate Follicles, and Human Follicle Maturation In Vitro) to develop surveys, focus group guidelines, and interviews while serving as an integrated link to interdisciplinary biomedical research in ovarian follicle harvesting, cryopreservation, maturation, and fertilization. Insights regarding the psychosocial impact of infertility on the lives of cancer patients and their families will be applied to the training, education, and advocacy mission of the Consortium.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058296.
Administrative Core
Site: Northwestern University
Principal Investigator: Teresa K. Woodruff, PhD
Rationale
The overarching purpose of the Oncofertility Consortium is to address the fertility threat posed by today’s highly effective cancer treatments in young men, women, and children and to serve as an authoritative voice for research, clinical practice, and training at the intersection of oncology, pediatrics, reproductive science and medicine, biomechanics, material science, mathematics, social science, bioethics, religion, policy research, reproductive health law, cognitive and learning science in a new discipline called oncofertility.
The development of this new discipline requires experts from a variety of fields in the creation of a common language and mechanisms to support communication and collaboration between Consortium members.
The interdisciplinary approach of the Oncofertility Consortium to the issue of fertility management after cancer is rather unique. Solving the distinct needs of cancer survivors is a complicated task that requires consistent collaboration and exchanges of ideas, successes and failures across a wide variety of specialties. Thus, the group must maintain its ability to cross disciplines in order to solve problems, continue developing solutions, and translate this progress into direct patient benfits . The work of the Administrative Core is as much about keeping the vision of the ultimate scope of the project in front of the individuals and groups involved and facilitating interaction among them as it is about traditional administrative governance.
The objectives of this program are to:
- Enable the interdisciplinary group and provide members with a strong, unified vision.
- Reduce barriers, encourage research, solve problems, maintain documents, and provide a robust intellectual environment with shared vision and an altruistic approach to credit and results.
- Ensure that administrative barriers do not interrupt the flow of intellectual energy and that the new ideas and data that are created at the boundaries of traditional disciplines are continuously fed back into the system.
Initial funding of this research was supported by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant 5UL1DE019587. Funding continues under the Center for Reproductive Health After Disease (P50HD076188) from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI)
Assessing the Benefits and Costs of Oncofertility Treatment
Site: Northwestern University and University of California, San Diego
Principal Investigators: David Dranove, PhD and Andrew Sfekas, PhD
Overview
This project is an economic analysis that weighs the costs and benefits of possible infertility and fertility preservation treatments in young adult women and parents with young children.
Objectives
- Understand the economic factors, decision-making rules, and risk-benefits analyses of accessing fertility conserving options when the outcomes (e.g., survival and infertility) are not known.
- Estimate the value of child bearing to 2 distinct populations: women of childbearing age (defined as 18-25) and parents of young girls (aged 5 to 17).
Indices of Scholarship
- Develop, validate, and implement a 4-stage survey to measure the direct medical costs as well as the indirect costs of choosing to pursue fertility preservation measures
- Following standard methods in cost-benefit analysis, evaluate the estimated value of child bearing using the concept of willingness to pay
- Based on variance in treatment costs and success rates outside of a clinical trial, construct a “break even” curve to depict the increase in probability of conception necessary to make treatment worthwhile at a particular cost
Consortium Support and Impact
This project draws on other parts of the Oncofertility Consortium in developing a social science inquiry that is informed by scientific innovation and insight. It relies on clinical and medical perspectives and advances and application of oncofertility technology (see Follice Cryopreservation, Bioengineering Primate Follicles, and Human Follicle Maturation In Vitro) to develop surveys, focus group guidelines, and interviews while serving as an integrated link to interdisciplinary biomedical research in ovarian follicle harvesting, cryopreservation, maturation, and fertilization. Insights regarding the psychosocial impact of infertility on the lives of cancer patients and their families will be applied to the training, education, and advocacy mission of the Consortium.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058296.
Bioengineering Primate Follicles
Site: Oregon Health & Science University
Principal Investigator: Richard L. Stouffer, PhD
Co-Investigators: Mary B. Zelinski, PhD; David M. Lee, MD
Rationale
While cancer eradication is the immediate and paramount goal for patients and their physicians, the increased chance of survival has led to efforts to ensure or restore the quality of life after cancer. Data indicates that fertility after treatment is a major concern of young female cancer patients; yet many women state that their concerns are not adequately addressed. Unlike young men who have a clinically proven option in sperm cryopreservation, the methods for fertility preservation in young women and girls remain experimental. This is due in part to the limited accessibility of the ovaries and difficulties in manipulating the larger oocyte.
A primary goal of the new oncofertility researcher is to merge the principles of tissue engineering and biomaterial science with ovarian biology to develop novel fertility preservation options for girls and young women who must undergo treatments that threaten their fertility. One strategy to prevent oocyte exposure to the toxic effects of therapy is to remove eggs or ovarian tissue for cryopreservation prior to therapy. Once treatment is complete, the goal is to either transplant thawed ovarian tissue or isolate the follicles from the tissue, mature them in vitro, and use them for in vitro fertilization.
In this project, translational studies of these techniques are being performed in nonhuman primates because they offer an easily manipulated, available system for determining if concepts originating from traditional research animals (mouse) are relevant to reproductive processes in primates, including humans. In addition, the nonhuman primate provides a means for assessing ethical and practical issues that cannot be studied in women or children.
The objectives of this project are to:
- Validate a matrix scaffold that supports the 3-D architecture of the primate follicle and permits the coordinated development of the follicle wall and oocyte in vitro
- Evaluate the role of gonadotropic hormones and growth factors in promoting the growth and maturation of primate follicles and their enclosed oocytes in vitro
- Optimize conditions for autotransplantation of ovarian cortex to readily accessible sites for coordinated follicle growth and oocyte maturation in vivo in primates
- Assess the fertilization and embryonic potential of primate oocytes derived from in vitro matured follicles and autotransplanted ovarian cortex
Key Experiments
- Mechanically isolate immature follicles from rhesus ovarian tissue and culture in various concentrations of alginate and alginate plus components of the ECM to determine the optimal scaffold for survival and growth of these follicles
- Define the extent and time course of neovascularization of fresh and cryopreserved ovarian tissue autografted to the arm and abdomen and determine if long term endocrine function is maintained post-transplantation
- Using in vitro fertilization, evaluate the reproductive potential of in vitro matured oocytes or oocytes retrieved from cortically transplanted tissue
Consortium Support and Impact
The successful execution of this project requires close collaboration with other members of the Oncofertility Consortium®. Specifically, this project will provide fresh ovarian cortical biopsies to cryobiologists for the development of effective methods for follicle cryopreservation. All advances in the nonhuman primate model will be rapidly translated to efforts using human ovarian tissue. The Biomaterials Core will provide alginate hydrogel matrices to support 3-D follicle culture, while the National Physicians Cooperative will provide a centralized medical network that will allow researchers at various sites and programs to quickly and easily access data and findings from this and other projects. Education of physicians and the public, and training of oncofertility specialists will be key elements to communicate the latest developments in primate follicle biology and their implications for women facing cancer.
Publications
Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011 Feb 28.
Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young and older adult, rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotropins and insulin. Reproduction, Aug. Epub 2010. PMID 20729335
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058294.
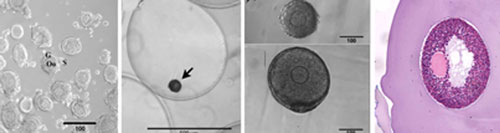
Biomaterials Core
Site: Northwestern University
Principal Investigator: Lonnie D. Shea, PhD
Rationale
 For folliculogenesis to properly occur, it is essential to maintain the intimate physiological connections between the oocyte and surrounding somatic cells. To recapitulate the 3-dimensionality of the ovary and maintain the appropriate size, shape, and architecture of the ovarian follicle while providing the necessary stimuli to direct cellular responses, a synthetic scaffold must serve as an in vitro mimic of the in vivo ovarian microenvironment.
For folliculogenesis to properly occur, it is essential to maintain the intimate physiological connections between the oocyte and surrounding somatic cells. To recapitulate the 3-dimensionality of the ovary and maintain the appropriate size, shape, and architecture of the ovarian follicle while providing the necessary stimuli to direct cellular responses, a synthetic scaffold must serve as an in vitro mimic of the in vivo ovarian microenvironment.
Previous work has demonstrated that the hydrogel alginate phenocopies the in vivo ovarian environment by maintaining follicular architecture and permitting combinations of diffusible, insoluble, and mechanical signals to influence the development of the follicle. Using this scaffold, murine ovarian follicles have been successfully matured in vitro to yield high-quality oocytes that were fertilized and used for the birth of live, viable offspring.
The application of biomaterials and tissue engineering to reproductive biology provides an enabling technology that is central to the Oncofertility Consortium®. The work of the biomaterials core directly supports the work of other Consortium members; in particular, growth of large nonhuman primate and human follicles that may have different requirements from mouse follicles, maintaining follicle architecture and facilitating handling during cryopreservation, and promoting engraftment and survival of primate follicles following autotransplantation of ovarian tissue.
The objectives of this project are to:
- Provide biomaterial support to the Oncofertility Consortium®
- Identify biomaterial properties and culture conditions to maximize primate follicle growth and transfer knowledge to appropriate projects
- Develop novel biomaterials that can be used to minimize tissue cryoinjury
- Engineer drug-releasing hydrogels to optimize ovarian cortical strip transplants
Key Experiments
- Assess the mechanical influence of the ovarian stroma on follicle development using the alginate culture system
- Perform thermal analysis and polymer molecular weight measurements before and after follicle cryopreservation to identify optimal alginate properties and cooling procedures for maximum survival of individual follicles and cortical strips
- Subcutaneously implant alginate hydrogels with immobilized ECM and angiogenic factors into the abdomen of mice and analyze cellular infiltration, identify cell types, and quantify blood vessel growth (vascularization) to gauge host tissue engraftment
Consortium Support and Impact
The Biomaterials Core provides critical service, support, and training to the other Oncofertility Consortium® projects. The exchange of biomaterials, development of techniques, training, and constant communication are central to the Core’s mission. In addition, there is reciprocal interaction as results from the individual projects feed into the Core to further optimize 3-D culture systems.
Publications
Shikanov A, Smoith R M., Xu M, Woodruff T K., Shea L D. Hydrogel Network Design Using Multifunctional Macromers to Coordinate Tissue Maturation in Ovarian Follicle Culture. Biomaterials. 2011. April; 32(10): 2524-31.
Silber S J., Woodruff T K., Shea D L. To Transplant or Not to Transplant – That is the Question. Cancer Treatment and Research. 2010; 156: 41-54. PMID: 20811824.
Smith, R M., Woodruff T K., Shea L D. Designing Follicle-Environment Interactions with Biomaterials. Cancer Treatment and Research. 2010; 156: 11-24. PMID 20811822.
Weiss MS, Peñalver Bernabé B, Bellis AD, Broadbelt LJ, Jeruss JS, Shea LD. Dynamic, large-scale profiling of transcription factor activity from live cells in 3D culture. PLoS One. 2010 Nov 17;5(11):e14026.PMID: 21103341
Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating Fibrin–Alginate Matrices For In Vitro Ovarian Follicle Development. Biomaterials Vol. 29 5476-85 Oct 30, 2009
West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, and Shea LD. The Mouse Follicle Microenvironment Regulates Antrum Formation and Steroid Production: Alterations in Gene Expression Profiles. Biology of Reproduction, 80, 432–439, 2009.
Xu M, Banc A, Woodruff TK, Shea LD. Secondary Follicle Growth and Oocyte Maturation by Culture in Alginate Hydrogel Following Cryopreservation of the Ovary or Individual Follicles. Biotechnology and Bioengineering, Vol. 103, No. 2, June, 2009.
West-Farrell ER, Xu M, Woodruff TK, Shea LD. Physical Properties of Alginate Hydrogels and Their Effects On In Vitro Follicle Development. Biomaterials Vol 30 4439-48 Oct 2007.
Woodruff TK and Shea LD. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod Sci. Vol 14 No 8 Suppl 6-10 Dec 2007
Xu M, PhD, Woodruff TK, and Shea LD. Bioengineering and the Ovarian Follicle. Cancer Treat Res. 2007;138:75-82.
Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of Extracellular Matrix Proteins Type I Collagen, Type IV Collagen, Fibronectin, and Laminin In Mouse Folliculogenesis. Histochem Cell Biol Vol 126 No 5 583-92 Nov 2006.
Kreeger PK, Deck JW, Woodruff TK, and Shea LD. The In Vitro Regulation Of Ovarian Follicle Development Using Alginate-Extracellular Matrix Gels. Biomaterials Vol 27 No 5 714-23 2006.
Kreeger PK, Fernandes NN, Woodruff TK, and Shea LD. Regulation of Mouse Follicle Development by Follicle-Stimulating Hormone in a Three-Dimensional In Vitro Culture System Is Dependent on Follicle Stage and Dose. Biology of Reproduction Vol 73 No 5 942-50 June 29 2005.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and PL1EB008542.
Developing a Health-Related Quality of Life Instrument for Adolescents Who Are Pursuing Fertility Preservation
Site: Moffitt Cancer Center and University of Florida
Principal Investigators: Caprice Knapp, PhD; Gwen Quinn, PhD
Overview
This study seeks to identify the fertility-related concerns of female adolescent cancer patients who have completed or are about to complete treatment.
Objectives
- To develop a Health Related Quality of Life (HRQOL) survey instrument for adolescents aged 12-18 who have been diagnosed with cancer.
- To conduct cognitive debriefing and determine if the items related to reproduction are appropriate and well understood by subjects.
Indices of Scholarship
- Review the literature and identify potential instruments that may be used to measure health related quality of life and reproductive concerns.
- Pilot test the reproductive concerns questions through interviews with daughter-parent paris in two age groups: 12-14 and 15-18. Eligible daughters include all female cancer patients who are either on or off treatment but healthy enough to participate as assessed by their oncologist. These interviews will assess clarity, sensitivity, and accuracy of the reproductive concerns items.
- Pilot test the reproductive concerns questions through four focus groups, two with girls between the ages of 12-14 and two with girls between the ages of 15-18. These focus groups will help identify acceptable language and content related to fertility discussions.
- Produce a guidebook to accompany the instrument based on interviews that have been completed thus far.
- Distribute a prompting tool for fertility preservation discussions such as a stress-ball in the shape of an egg.
Consortium Support and Impact
This project draws on other parts of the Oncofertility Consortium in developing a social science inquiry that is informed by scientific innovation and insight. It relies on clinical and medical perspectives and advances and application of oncofertility technology (see Follice Cryopreservation, Bioengineering Primate Follicles, and Human Follicle Maturation In Vitro) to develop surveys, focus group guidelines, and interviews while serving as an integrated link to interdisciplinary biomedical research in ovarian follicle harvesting, cryopreservation, maturation, and fertilization. Insights regarding the psychosocial impact of infertility on the lives of cancer patients and their families will be applied to the training, education, and advocacy mission of the Consortium.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058296.
Education & Outreach Core: Providing Reproductive and Endocrine Awareness to Diverse Communities
Site: Northwestern University
Investigators: Teresa Woodruff, PhD; Ellen Wartella, PhD, and Eric Patrick
The Education and Outreach Core of the SCCPIR Center for Reproductive Health After Disease provides Reproductive and Endocrine Awareness to Diverse Communities. It creates a process to communicates the research activities and emerging data of the Center to two important audiences:
- The clinicians who treat patients diagnosed with diseases or undergoing treatments that threaten their future reproductive health, and
- The public, who should have a general understanding of their own reproductive and endocrine health and the risk of disease-related or iatrogenic reproductive dysfunction.
For Clinicians and Researchers
The Education and Outreach Core conveys information to the clinical community regarding the reproductive and endocrine damage caused by specific diseases and treatments. To disseminate clinical information and emerging Center research findings to providers across the United States, the Core hosts quarterly Virtual Grand Rounds, through which attendees can view and listen to experts in the fields of disease, reproduction, endocrinology, and fertility preservation and interact with presenters in real time. Many fertility providers and health care clinicians have detailed questions regarding how to implement a comprehensive fertility preservation program, thus the Core hosts Clinic 101, an annual small-group training course, to give providers the resources and tools to develop their own fertility preservation and endocrine health program or to strengthen their existing program.
For the Public
A solid understanding of reproductive biology is important to ensure that all people can make fully informed decisions about their future reproductive health. Thus, public education is a critical function of the Core to disseminate information to male and female patients, their partners, and their parents. In addition, outreach to children and teenagers is necessary to provide them with a basic understanding of reproductive biology, and a basis for conceptualizing advanced reproductive and endocrine concepts as the future researchers, clinicians, and patients of tomorrow. To ensure these concepts are taught to young people, the core is developing animated video training modules targeted to young boys and girls. By providing reproductive education and outreach to this diverse set of stakeholders—providers, patients, partners, parents, and the public—we can be assured that, together with their physicians, patients and the public will have the ability to make complex decisions about their reproductive and endocrine health that will affect the rest of their lives.
Engineered Environments for Ovarian Follicle Transplantation
Site: Northwestern University
Principal Investigator: Lonnie D. Shea, PhD
Rationale
Compared to their age-matched peers, the five-year relative survival rate of cancer patients is now at 68%, improved from 50% thirty years ago. At least one in 250 women of reproductive age are cancer survivors. Today, 90% of these young women will be cured of their cancer. Unfortunately, the chemotherapies that save their life are fertility-threatening; in particular, alkylating agents and platinum-based drugs are highly associated with post-treatment infertility as they cause DNA damage to the oocytes that comprise the ovarian reserve.
Most young women with cancer are highly interested in trying to preserve their fertility so they might have children in the future. The cryopreservation and autotransplantation of ovarian tissue is emerging as a powerful approach for preserving fertility for patients that are losing ovarian function. Ovarian tissue transplantation has preserved fertility (at least 22 live births to date); however, for cancer patients, transplantation may not be possible due to the risk of re-seeding disease.
The objectives of this project are to:
- Refine the procedures for follicle isolation to enable large-scale recovery of primordial follicles, and subsequently investigate the engraftment and function by transplanted follicles within a range of biomaterials.
- Investigate strategies to modulate the initial recruitment of follicles that would deplete the ovarian reserve, and thereby enhance the duration of graft function.
- Investigate the transplantation of follicles from post-pubertal animals, and determine the contribution of age and obesity of the recipient on the engraftment and function of transplanted follicles.
- Investigate the transplantation of ovarian follicles from mice with metastatic disease, which simulates the clinical situation of re-seeding cancer cells.
Read about Dr. Shea’s previous project: The Oncofertility Consortium Biomaterials Core
Fertility Measures After Cancer
Site: University of Pennsylvania
Principal Investigator: Clarisa Gracia, MD
Rationale
There are currently over 250,000 survivors of childhood cancer in the U.S. While recent advances in therapeutic technologies have led to an increase in cancer survivorship, these powerful therapies may also lead to impaired fertility and premature ovarian insufficiency. While reduced reproductive potential is well documented in adolescents and young adults with cancer, little is known about these patients’ hypothalamic-pituitary-gonadal function prior to, during, and after treatment. And though serum hormone and ultrasound measures are routinely used as surrogate markers of fertility in adult women prior to and after treatment, they have yet to be validated in pediatric cancer patients.
This project aims to develop means for early detection of decreased fertility potential in order to identify individuals who would benefit from application of existing assisted reproductive technologies and/or emerging novel fertility preserving methods such as ovarian tissue cryopreservation. The results from this project will serve as preliminary data for the establishment of a long-term cohort study of pediatric cancer survivors to examine the association between ovarian function measures, pregnancy rates, pregnancy outcomes, and the occurrence of premature menopause.
The objectives of this project are to:
- Determine whether existing and novel surrogate measures of infertility potential differ between female pediatric cancer patients and healthy controls.
- Determine whether a relationship exists between surrogate measures of fertility potential and the dose of alkylating agents received by female pediatric cancer survivors.
- Assess changes in existing and novel surrogate measures of fertility potential during and after chemotherapy in pediatric cancer patients.
Key Experiments
- After recruiting and interviewing a prospective cohort of pediatric cancer survivors and age-matched healthy controls, a comparison of ovarian function as a whole and based on dose of alkylating agent will be conducted. Various markers of ovarian function will be measured in order to establish their prognostic value for predicting impaired fertility and improving counseling methods on fertility preserving techniques
- In pediatric patients, evaluate endocrine changes in the hypothalamic-pituitary-gonadal axis before and after chemotherapy with alkylating agents using various markers of ovarian function
Consortium Support and Impact
In order to recruit the appropriate number of patients for these studies and to take advantage of oncofertility mentorship, there must be close collaboration with the National Physicians Cooperative. No study to date has conducted such a comprehensive evaluation of fertility potential in pediatric and young adult cancer survivors; therefore, this project provides an opportunity for close collaboration with social science researchers to determine the biosocial perspective of these patients. The impact of pediatric cancer treatments on fertility potential will be directly informative to those studying the impact of treatments in the adult female and vice versa. Education of patients and physicians and training of oncofertility specialists will also be necessary to ensure an understanding of the impact of infertility in pediatric patients and that appropriate technology is translated into bedside care.
Publications
Gracia, C R. Reproductive Health After Cancer. Cancer and Treatment Research. 2010; 156: 3-9. PMID: 20811821.
Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, Freeman EW, Gracia CR, Demichele A. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2009 Nov 13.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and KL1CA133839.
Follicle Cryopreservation
Site: Oregon National Primate Research Center
Principal Investigator: Mary B. Zelinski, PhD
Co-Investigator: Richard R. Yeoman, PhD
Rationale
While fertility preservation for males has been available for several decades using sperm cryopreservation, attempts to similarly preserve mature female oocytes and ovarian tissue have been more challenging. Tissue damage during the freezing and thawing process has the potential to irreversibly disrupt the 3-dimensional structure of the follicle necessary for the development of a fully viable mature oocyte. The goal of this research is to provide the direction necessary by testing existing technologies that represent the state-of-the-art in cryopreservation of ovarian tissue samples in nonhuman primates. In addition, novel methods that will improve tissue viability after thaw as well as minimize operator time and effort will be developed.
Experimental endpoints include successful in vitro maturation of follicles, fertilization of resulting mature oocytes, embryo development and establishment of pregnancy. After rigorous testing in nonhuman primates, the findings will be rapidly applied to the cryopreservation of human ovarian tissue in order to expand fertility preservation options for women at the time of diagnosis of cancer and other serious diseases.
The objectives of this project are to:
- Compare two methods of ovarian tissue cryopreservation (slow freeze vs. vitrification) in the nonhuman primate, and assess follicle and oocyte viability in all stages of follicle maturity
- Assess the fertilization and embryonic potential of primate oocytes derived from cryopreserved ovarian tissue and cumulus-oocyte complexes
- Adapt new cryo-technology under development around the globe to the nonhuman primate model
Key Experiments
- Compare vitrification to slow freeze methods in rhesus monkey ovarian tissue by assessing a) histology of tissue preservation; b) in follicle maturation (IFM), i.e., isolation of individual secondary follicles to monitor survival, growth and functional characteristics using the encapsulated 3-dimensional (3D) culture system recently established in the nonhuman primate; and c) isolation of the matured oocyte and assessment of fertilization, early embryonic development, and ultimately production of live offspring.
- Compare vitrification to slow freeze methods in cumulus–oocyte complexes (COCs) derived from small antral follicles in rhesus monkey ovarian tissue and assessing oocyte maturation in vitro, fertilization, early embryonic development and ultimately production of live offspring.
- Develop novel cryopreservation methods based on cutting-edge technologies derived from the cryobiology field (i.e., novel cryoprotectants) in rhesus monkey ovarian tissue and COCs
- Test methods for transporting ovarian tissues (currently used for tissue transplantation) rapidly between sites for optimal preservation of morphology and function
Consortium Support and Impact
The successful execution of this project and eventual application of preservation technology will require close collaboration with other members of the Oncofertility Consortium. Specifically, the project requires close cooperation with the team working to improve methods to mature primate follicles in vitro and the Biomaterials Core. In addition, a primary goal of the National Physicians Cooperative is to ultimately translate the results of these studies to the preservation of human ovarian tissue and follicles. While the human application of methods to cryopreserve and recover ovarian tissue will fill a significant need in fertility preservation in women, it is also expected to lead to new social, legal, and ethical concerns that will impact how patients and physicians discuss infertility (see Social Science and Oncofertility), particularly with regard to pediatric cancer patients. Education of patients and physicians and training of oncofertility specialists also be necessary to ensure that cutting-edge procedures associated with follicle removal and storage become part of routine cancer care worldwide
Publications
Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011 Jun 24.
Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011 Feb 28.
Jin, S, Lei, L, Shea, LD, Zelinski, MB, Stouffer, RL, and Woodruff, TK. Markers of growth and development in primate primordial follicles are preserved after slow cryopreservation. Fertility and Sterility, 2010.
Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus-Oocyte Complexes from Small Antral Follicles During the Early Follicular Phase of Menstrual Cycles in Rhesus Monkeys Yield Oocytes That Reinitiate Meiosis and Fertilize In vitro. Biol Reprod 83:525-532, 2010. PMID 20519694.
Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, and Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follico development and transplantation: implications for fertility preservation. Human Reproduction Update, 2010.
Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, and Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation (Chinese excerpt). Human Reproduction Update, Vol.16, No.4 pp. 395–414, 2010.
Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young and older adult, rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotropins and insulin. Reproduction, Aug. 2010. PMID 20729335
Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, and Zelinski MB. Encapsulated Three-Dimensional Culture Supports Development of Nonhuman Primate Secondary Follicles. Biology of Reproduction. (3):587-94 Sep.8, 2009
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058293.
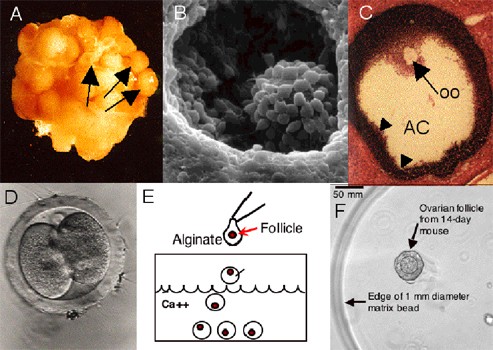
Human Follicle Maturation in Vitro
Site: Northwestern University
Principal Investigator: Teresa Woodruff, PhD
Rationale
While advances in radiation and chemotherapy have improved survival rates, these treatments may also permanently impact the reproductive capacity of cancer survivors. Nevertheless, fertility preservation technologies have made the phrase “families after cancer” a reality for many young women.
The role of the new oncofertility specialist is to bridge the gap between oncology and reproductive medicine and help patients navigate the available fertility preservation options and provide individual solutions based on factors such as age, partnership status, time constraints prior to cancer treatment, planned treatment regimen and overall medical condition. To provide this information to patients effectively, it is necessary to first evaluate the reproductive axis and its response to cancer treatment in a rigorous manner.
Unfortunately, there are few options for fertility preservation that can be offered to children and young girls diagnosed with cancer and other serious diseases. Development and application of new technologies that can be offered regardless of age is necessary to expand the possibility of having a family after cancer to to all female patients diagnosed with cancer.
The objectives of this project are to:
- Assess ovarian function and develop methodology for predicting the risk of impending premature ovarian insufficiency based on a young woman’s cancer treatment plan
- Evaluate the toxicity of existing and new chemotherapeutic agents on in vitro follicle culture survival and maturation in order to determine a relative fertility risk
- Optimize current in vitro follicle culture methods in established murine and non-human primate models and translate the successful system into methods that support human follicle growth, steroid production and oocyte maturation in vitro
Key Experiments
- Perform a longitudinal study of women with a new cancer diagnosis to measure their ovarian reserve before, during and after cancer treatment using ultrasound imaging of the ovaries, serum hormone levels, ovarian blood flow and follicle attrition
- Add varying doses of the main types of cancer therapeutic agents, and any newly developed drug therapies, to the established murine follicle culture model for the identification of trends in the type of in vitro damage caused to the follicle
- Create the optimal in vitro environment for growth of human follicles by collectively providing the proper mechanical support, growth factor and hormone contribution exposure in a time and stage dependent manner
Consortium Support and Impact
The successful execution of this project and eventual translation of results to the clinic require close collaboration with other members of the Oncofertility Consortium. Specifically, cooperation with the Biomaterials Core and two other Consortium projects that are focused on new methods for follicle cryopreservation and maturation of ovarian follicles in nonhuman primates will be paramount to achieving project goals. In addition, the ability to recruit patients through the National Physicians Cooperative will provide sufficient materials and statistical power to correlate treatment and outcomes data in a comprehensive and thorough manner and to rapidly disseminate new developments for clinical application. Other Consortium projects will be impacted by the human application of in vitro follicle maturation. This work is expected to open up new avenues of social and ethical inquiry in oncofertility, particularly with regard to its application in pediatric cancer patients. Education of patients and physicians and training of oncofertility specialists will also be necessary to ensure that new fertility-conserving techniques become part of routine cancer care worldwide.
Publications
Barrett SL, Woodruff TK. Gamete Preservation. Cancer Treatment and Research. 2010; 156: 25-39. PMID: 20811823.
Barrett SL, Shea LD, Woodruff TK. Noninvasive Index of Cyrorecovery and Growth Potential for Human Follicles In Vitro. Biology of Reproduction, 2010.
Lei L, Jin S, Mayo KE, and Woodruff TK. The Interactions Between the Stimulatory Effect of Follicle-Stimulating Hormone and the Inhibitory Effect of Estrogen on Mouse Primordial Folliculogenesis. Biology of Reproduction, 82, 13-22, 2010.
Jin S, Lei L, PhD, Shea LD, PhD, Zelinski MB, Stouffer RL, and Woodruff TK. Markers of growth and development in primate primordial follicles are preserved after slow cryopreservation. Fertility and Sterility, 2010.
Jin SY, Lei L, Shikanov A, Shea LD, and Woodruff TK. A Novel Two-Step Strategy For In Vitro Culture Of Early-Stage Ovarian Follicles In The Mouse. Fert. Ster. 2010.
West ER, Shea LD, and Woodruff TK. Engineering the Follicle Microenvironment. Semin Reproduction Med. Vol 25 No 4 287-99 July 2007
West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, and Shea LD. The Mouse Follicle Microenvironment Regulates Antrum Formation and Steroid Production: Alterations in Gene Expression Profiles. Biology of Reproduction, 80, 432–439, 2009.
Xu M, Kreeger PK, Shea LD, and Woodruff TK. Tissue-Engineered Follicles Produce Live, Fertile Offspring. Tissue Engineering Vol 10 2739-46 Oct 13 2006
Xu M, West ER, Shea LD, and Woodruff TK. Identification of a Stage-Specific Permissive In Vitro Culture Environment for Follicle Growth and Oocyte Development. Biology of Reproduction Vol 75 916-923 Sep 6 2006
Xu M, Barrett SL, West-Farrell ER, Kondapalli LA, Kiesewetter SE, Shea LD, and Woodruff TK. In Vitro Grown Human Ovarian Follicles From Cancer Patients Support Oocyte Growth Human Reproduction. Vol.24, No.10 2531-40 Oct. 24, 2009
Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshefeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In Vitro Oocyte Maturation and Preantral Follicle Culture from the Luteal Phase Baboon Ovary Produce Mature Oocytes. Biol Reprod. 2011 Apr;84(4):689-97. 2010 Dec 1. PMID: 21123815
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058295.
Humanities Scholars
Fertility preservation technologies raise a variety of ethical, social, and legal challenges and concerns. This section of the website is devoted to those working in the humanities who research these issues from disciplines such as bioethics, history, literature, philosophy, religions, law, and the arts. Humanists have been involved in the Oncofertility Consortium since its inception, providing parallel scholarship to accompany scientific and technological developments, proving an integral part of the Oncofertility Consortium team.
This project evaluates the ethical and legal issues surrounding the use of reproductive technologies in cancer patients, with a special emphasis on the implications for pediatric patients and their families.
For a complete description of the humanities projects currently supported by the Oncofertility Consortium, please visit our Humanities, Social Science, and Oncofertility page.
Publications:
- Consistency in Insurance Coverage for Iatrogenic Conditions Resulting from Cancer Treatment Including Fertility Preservation
- Medical Hope, Legal Pitfalls: Potential Legal Issues in the Emerging Field of Oncofertility
Presentations:
In July 2009, the Oncofertility Consortium hosted a robust summit that brought together scholars from the humanities, social sciences, and the basic and clinical sciences to examine the complex issues raised by recent developments in the field of oncofertility and to provide interdisciplinary perspectives to help shape the understanding and delivery of this new field. Papers and presentations from this summit are available in our Virtual Library.
Dr. Paul Lauritzen has also prepared a podcast titled which exlpores oncofertility from the Catholic perspective. This insightful presentation offers a unique look into the implications of developing fertility preservation technologies from the perspective of an accomplished religious scholar.
In early 2011, the Chicago Council for Religious Clergy met with the Oncofertility Consortium to begin a discussion between scientists, ethicists, and a variety of religious traditions about the evolving technologies in fertility preservation. The presentations can be viewed here and additional materials will be added as the conversation continues:
- Oncofertility: The Preservation of Fertility Options for Young People with Cancer – Teresa K. Woodruff, Ph.D.
- Conversations in the Future Tense: Ethical Issues in Ovarian Tissue Salvation and Egg Generation – Laurie Zoloth, Ph.D.
The Oncofertility Consortium recognizes that society is always in flux, and thus understands that humanities research is continually evolving. If you are a humanist interested in pursuing a novel research aim related to oncofertility or have questions or comment about our existing research project, please contact us.
Learning Modules in Oncofertility
Principal Investigator: Kemi Jona, PhD
Development of an Oncofertility Curriculum for high school girls.
 The Oncofertility Saturday Academy was founded in 2007 to expose high school students to laboratory and medical science research in the fields of cancer, fertility, and oncofertility. The keystone educational program of the Women’s Health Science Program, the program educates students at four locations around the country: Chicago, IL; San Diego, CA; Phildadelphia, PA; and Oregon (with a fifth in development in Denver, CO).
The Oncofertility Saturday Academy was founded in 2007 to expose high school students to laboratory and medical science research in the fields of cancer, fertility, and oncofertility. The keystone educational program of the Women’s Health Science Program, the program educates students at four locations around the country: Chicago, IL; San Diego, CA; Phildadelphia, PA; and Oregon (with a fifth in development in Denver, CO).
The programs include laboratory learning experiences in mouse ovary dissections, ovarian follicle encapsulation, DNA analysis, immunohistochemistry, in vitro fertilization, and developmental processes of sea urchins and frogs. Clinical learning experiences include performing breast and pelvic exams on simulation models, operating the daVinci surgical robot under the supervision of surgeons, and performing embryology techniques. In addition, bioethicists facilitate and guide the high school students to apply an ethical framework to discuss the various perspectives connected to the field of oncofertility.
Learn more about OSA in Chicago
Learn more about OSA in San Diego
Publications
Faurot M, Woodruff TK. The Oncofertility Saturday Academy: A Paradigm to Expand the Educational Opportunities and Ambitions of High School Girls. Cancer Treatment and Research. 2010; 156; 321-44. PMID: 20811845.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL5CA133836.
Measuring and Modifying the Human Follicle Environment to Improve In Vitro Egg Quality
Site: Northwestern University
Principal Investigator: Teresa Woodruff, PhD
Rationale
Life-preserving strategies in the face of a cancer diagnosis, including radiation, surgery, and chemotherapy, can compromise nearly all aspects of the female reproductive axis resulting in infertility, subfertility, or premature menopause. In addition, several conditions and disease states can have a similar consequence.
Fortunately for females, the ovary has the potential for diverse fertility preservation options ranging from standard (i.e. IVF, ICSI, embryo banking) to investigational (ovarian tissue cryopreservation, ovarian transplant).
In vitro follicle growth is another emerging technology that has tremendous promise for a subset of individuals who have blood-borne malignancies, cannot tolerate supraphysiological hormone levels, can not delay treatment, or do not have a sperm donor. Tremendous success has been achieved in performing IVFG in the mouse to the degree that live births have been achieved using gametes derived from nearly all methods attempted to date. The translation of this technology to large mammalian species, however, has not been trivial due to significant differences in factors such as follicle size, development, metabolic requirements, and physical niche.
The objectives of this project are to:
- In this project, we will develop “smart” biomaterials that will provide human follicles with the ability to self-regulate their physical environment as they grow to terminal stages of development. We anticipate that such an environment will maintain the coordinated growth of the oocyte and its companion granulosa cells, which is required for optimal endocrine function and gamete quality.
- We will also develop new non-invasive endocrine measures of follicle health that, through a precise algorithm, could be used in association with conventional steroid and peptide assays to predict the maturity of individual follicles.
- Follicles developed to the right stage of maturity will contain the highest quality gamete, and we will use innovative nanonewton force measurements to quantitatively understand the mechanisms by which a chromosomally normal egg is generated both in vivo and in vitro during meiosis.
These experiments have broad implications on human health as a chromosomally normal egg is a fundamental pre-requisite to create a healthy offspring. Our aims are based on experiments that integrate three model organisms – mouse, cow, and human – and span multiple disciplines – reproductive medicine, cell and molecular biology, bioengineering, and biophysics. This union will allow us to advance our understanding of human reproductive biology in a quantitative way, and at the same time provide key insights into the possible clinical translation of in vitro follicle culture systems to preserve fertility in young patients with cancer or other reproductive disorders.
Read about Dr. Woodruff’s previous project: Preservation and Growth of Human Follicles
Medically-based Protection of the Ovarian Reserve Against Anti-Cancer Therapy
Site: Northwestern University
Rationale
The five-year relative survival rate of cancer patients has improved from 50 to 68% in the last thirty years. The survival rate of pediatric cancers has reached 80%, and the NCI predicts that 1 out of every 250 adults will be a survivor of childhood cancer by 2015. As the number of cancer survivors increases, so does the concern for their quality of life post-treatment.
One of the most serious problems associated with radiation and chemotherapy is the off-target effect on germ cells. For young women, this is a particularly serious concern because the immature oocytes that comprise the ovarian reserve are highly sensitive to chemo/radiation therapies.
Since the ovarian reserve represents ALL the follicles available for future fertility as well as endocrine function, the loss of immature oocytes and subsequent sex steroid deficiency have significant physical and psychological consequences. The goal of this project is to better understand the mechanisms associated with survival and death of immature oocytes caused by cancer treatments, and in doing so, identify “neo-adjuvant” medical approaches for preserving fully functional ovaries in young women undergoing treatment.
The overall hypothesis of this proposal is that the balance between survival and death of oocytes in primordial and primary follicles is controlled by the interaction between p53 family members, and thus the p53 family members are targets for fertoprotective agents.
The objectives of this project are to:
- Elucidate the mechanism through which p53 family members are activated by chemotherapy.
- Dissect the molecular pathways involved in the death of immature oocytes by applying in vitro culture and subrenal grafting of mouse ovaries to genetically engineered mouse models.
The results of these proposed studies will be critically important for understanding the mechanisms that underlie physiologic and iatrogenic follicle death and may reveal new strategies for protecting the fertility of young women diagnosed with cancer.
National Physicians Cooperative to Preserve Fertility for Female Cancer Patients
For more information, please visit Oncofertility Professional Engagement Network.
Pilot Project Program
As the field of oncofertility continues to advance, the Oncofertility Consortium seeks to support various high-risk, high-gain initiatives in order to facilitate the launch of early-stage ideas into future grant-funded projects. Applications for pilot projects are evaluated each year, with only a few awarded with financial support and access to Oncofertility Consortium resources. A total of 8 pilot projects have been backed by the Oncofertility Consortium, with additional projects planned each year.
Funded Projects, Current and Past:
- Improving Tamoxifen Adherence by Addressing Fertility Concerns
- A Novel Approach to Assess Direct and Estradiol-Mediated Effects of Ovulation Induction Regimens and Breast Cancer Development
- The Male Perspective: Survey for Preservation of Adolescent Reproduction
- Oncofertility Communication Initiative: Assessing the Short Documentary Format for Oncofertility Communications
- Development of Blood Spot Assays for Long-term Longitudinal Studies of Reproductive Status of Cancer Survivors
- Deriving Oocytes from Embryonic Stem Cells
- The Effects of GDF9 Levels on TZP Reorganization and Oocyte Competence in Growing Follicles Cultured in Alginate
- Identification of Developmental Markers and Culture Environments
- Role of GDF-9 in Human Folliculogenesis
- Does Gleevac Block Programmed Cell Death in Primary and Primordial Mouse Follicles Via the p63 Pathway?
- Gene Expression in Small Antral Follicles Derived in Vivo and From Encapsulated 3-Dimensional Culture in Macaques
- Comparing Infertility and Cancer Research, Development and Social Understandings
- The Road to Adulthood: How Do Young Adults Make Health and Fertility-Related Decisions
______________________________________
1. Improving Tamoxifen Adherance by Addressing Fertility Concerns
Site: Northwestern University
Principal Investigator: Jacqueline S. Jeruss, MD, PhD
Study Description
The study objective is to evaluate physician- and patient-level factors, including fertility, concerns that affect adherence to tamoxifen for young women diagnosed with breast cancer. An estimated 23,000 women under age 45 are diagnosed with breast cancer annually, of whom roughly 70% have estrogen-receptor positive (ER+) breast cancer. For these women, a 5-year course of daily tamoxifen results in a 47% reduction in annual recurrence risk and a 26% reduction in annual mortality. Despite such substantial benefits, adherence and persistence are low: 20% of patients fail to reach the optimal adherence threshold during the first year of treatment, and at 5 years, up to 50% of patients have discontinued treatment. Young women consistently have been shown to have lower rates of adherence to hormonal therapy when compared to women between the ages of 50-70. It is necessary to address both clinician and patient barriers to tamoxifen adherence. We hypothesize that, for young breast cancer patients, a unique set of patient- and provider-level factors, including fertility concerns, contributes to the poor tamoxifen adherence observed in this patient population.
2. A Novel Approach to Assess Direct and Estradiol-Mediated Effects of Ovulation Induction Regimens and Breast Cancer Development
Site: Northwestern University
Principal Investigator: Jacqueline S. Jeruss, MD, PhD
Study Description
The major goal of this project is to determine if ovulation induction through medical intervention causes breast epithelial cells to progress to neoplasia, either directly by inducing proliferation or indirectly by increasing stromal angiogenesis in a 3-dimensional culture system. In addition, investigators are determining if prior infertility treatment increases the risk of breast cancer in a cohort of women with BRCA 1/2 mutations or a family history of breast cancer.
Progress and Results
Clomiphene, commonly used as a treatment for infertility, acts by blocking the estrogen receptor (ER), though it has been hypothesized that this drug also effects cell proliferation in vitro by estrogen independent mechanisms. To analyze the effects of clomiphene treatment on breast cancer development we used 3 well-established breast cancer cell lines. MCF-10A cells, which approximate normal immortalized mammary epithelium and are ER negative, showed a slight increase in colony area in response to estrogen and a slight increase in colony number in response to clomiphene during 10 days of treatment. MCF-7 cells, which are estrogen receptor (ER) positive and well-differentiated breast cancer cells, showed a significant increase in colony area in response to estrogen treatment and a slight increase in colony number in response to clomiphene. HCC 1937 cells, which are ER negative and harbor a BRCA1 mutation, showed a slight increase in colony number n response to estrogen. Proliferation assays, 3D culture, and cell cycle analysis studies are in progress to determine the response of these cells to clomiphene, FSH, estradiol, and hCG. We will also use a mouse model with mammary epithelial hyperplasia to assess how infertility treatment affects the progression of hyperplasia to malignancy. Lastly, we continue to accrue BRCA 1/2 carriers or patients with a strong family history of breast cancer to determine the risk of developing breast cancer following infertility treatment. Publications from this and related research from Dr. Jeruss are:
Jeruss J S. Discussing Fertility Preservation with Breast Cancer Patients. Cancer Treatment and Research. 2010; 156: 461-6. PMID: 20811855.
Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier JN, Buzdar AU, Hortobagyi GN, Hunt KK. Staging of breast cancer in the neoadjuvant setting. Cancer Res. 2008 Aug 15;68(16):6477-81. PMID: 18701468.
Weiss MS, Peñalver Bernabé B, Bellis AD, Broadbelt LJ, Jeruss JS, Shea LD. Dynamic, large-scale profiling of transcription factor activity from live cells in 3D culture. PLoS One. 2010 Nov 17;5(11):e14026. PMID: 21103341.
3. The Male Perspective: Survey for Preservation of Adolescent Reproduction
Site: Northwestern University
Principal Investigator: Robert Brannigan, MD
Study Description
Understanding and overcoming the barriers to fertility preservation is essential to optimize the reproductive health of young cancer patients. The overall aim of this project is to develop and execute a national survey (Survey for Preservation of Adolescent Reproduction, SPARE) of pediatric oncologists to assess perspectives on fertility preservation in both pre-pubertal (age 1-12) and pubertal (age 13-18) cancer patients, including willingness to discuss fertility, knowledge of current fertility preservation methods, and awareness of the American Society of Clinical Oncology published fertility preservation recommendations (ASCOR) regarding sperm cryopreservation strategies and referral to a fertility specialist. Through SPARE, we sought to obtain post-ASCOR data from pediatric oncology specialists nationwide.
Progress and Results
A total of 209 respondents, primarily consisting of pediatric oncologists, responded to the SPARE survey, providing data on both male and female pediatric oncology fertility preservation knowledge, attitudes, and practices. Our work on SPARE revealed that fertility preservation in both male and female adolescents is hampered by a lack of knowledge among treating physicians of the American Society for Clinical Oncology recommendations on this topic, as well as numerous barriers to the delivery of fertility preservation case. This project also provided insight into the gender disparity that exists in the approach that clinicians utilize in treating male and female adolescents. We conclude that pediatric oncologists are strongly motivated to preserve fertility in male pediatric cancer patients but barriers to semen preservation and referral to fertility specialists exist. Additional promotion of the ASCO fertility preservation recommendations is essential to optimize reproductive health of young cancer patients. This pilot project sucessfully resulted in a publication, as follows:
Köhler TS, Kondapalli LA, Shah A, Chan S, Woodruff TK. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. Brannigan RE. J Assist Reprod Genet. 2010 Nov 26.
4. Oncofertility Communication Initiative: Assessing the Short Documentary Format for Oncofertility Communications
Site: Northwestern University
Principal Investigator: Barbara O’Keefe, PhD
Co-Investigator: Sean Zehnder, PhD
Study Description
Video portrayals of cancer patients offer special opportunities to shape the way audiences think about issues related to a diagnosis of cancer. We hypothesize that video portrayals of patients that dramatize their maturation into complex, multidimensional human beings will lead audiences of all types to cognize other cancer patients in more complex, multidimensional, and historicized ways, and that this broader framing will in turn change decision-making. The purpose of this project is to pilot both (a) the use of short documentary films for increasing the salience of fertility issues in decision-making about cancer treatment (oncofertility) and (b) methods for designing, disseminating, and evaluating messages about oncofertility. A long-term goal is to use the knowledge gained via this pilot to build a sustained research and development program on oncofertility communications.
Progress and Results
The first part of our pilot project was to produce three short documentaries about cancer survivors and their issues they have faced related to having biological children. Two Chicago-based professional videographers were hired and helped to produce a high definition video that portrays individual patient experiences with cancer. These videos tells to story of three unique cancer survivors: Gayle, a survivor of adult cancer who opted to participate in a new research protocol with the hope of one day being able to conceive another child; Wesley, a marathoner and business man who was diagnosed with Hodgkin’s Lymphoma as a young man; and Connie, a survivor of childhood cancer who is now struggling with infertility caused by her cancer treatment. These videos will help to develop evidence-based guidelines for web communication with patients and others and assist in the promotion of information about oncofertility. View the videos.
5. Development of Blood Spot Assays for Long-term Longitudinal Studies of Reproductive Status of Cancer Survivors
Site: Northwestern University
Principal Investigator: Teresa K. Woodruff, PhD
Co- Investigators: Clarisa Gracia, MD, Laxmi Kondapalli, MD, and Thomas McDade, PhD
Study Description
The blood spot assay is a method for collecting samples for hormone measurements. Dried blood spot (DBS) samples, which are collected by a minimally invasive finger prick, require little collection time, no post-collection processing, have low biohazard risk, and facilitate sample storage and transport. In this pilot project, DBS assays will be developed to measure markers of reproductive function (inhibin B, AMH, and FSH). These hormone profiles can then be used to assess reproductive potential in cancer patients. The aims of this project are to:
- Validate measurements of inhibin B, AMH and FSH in media and dried blood spots
- Assess inhibin B, AMH and FSH in pools of serum samples collected from early follicular phase, mid follicular phase and luteal phase
- Assess inhibin B, AMH and FSH in blood spots taken during one complete menstrual cycle and compare the results to a serum-based assay
Progress and Results
We have developed an enzyme immunoassay protocol for AMH using DBS derived from finger stick whole blood samples, and are currently working to improve the assay sensitivity. We have also begun collecting a set of 100 matched plasma/dried blood spot samples from menstruating reproductive aged women at the University of Pennsylvania. These samples will allow us to compare results obtained from DBS with gold-standard clinical methods using serum/plasma from venipuncture. Using Bland-Altman plots and regression analyses, we will inspect for agreement and bias across the assay range and generate a conversion formula for calculating plasma equivalents based on DBS results.
6. Deriving Oocytes from Embryonic Stem Cells
Site: Oregon National Primate Research Center
Principal Investigator: Shoukhrat M. Matalipov, PhD
Co-Investigator: Mary B. Zelinski, PhD
Postdoctoral Fellow: Araceli Valle Prieto
Project Description
Recent research suggests that embryonic stem cells (ESCs) could serve as a source of viable gametes, since ESCs can proliferate indefinitely in an undifferentiated pluripotent state and, when allowed, can differentiate into any cell type of an adult body. The goal of this proposal is to evaluate the potential of monkey ESCs to contribute to the female germ line lineage and to explore the feasibility of deriving functional oocytes suitable for use in assisted reproductive technologies (ARTs). We hypothesize that primate ESCs derived by somatic cell nuclear transfer can form female germ cells and gametes upon spontaneous or directed differentiation. In this pilot project, we propose:
- To develop optimal culture conditions for directed differentiation of monkey ESCs to the female germ cell lineage
- To evaluate three-dimensional (3-D) culture for directed differentiation of monkey ESC-derived follicles to functional oocytes.
Progress and Results
We have developed genetically tagged monkey ESCs under the control of germ cell-specific promoters that will allow us to detect and select early germ cells upon induced differentiation.
7. The Effects of GDF9 Levels on TZP Reorganization and Oocyte Competence in Growing Follicles Cultured in Alginate
Site: Northwestern University
Principal Investigator: Teresa K. Woodruff, PhD
Co-Investigators: Susan L. Barrett, MS, PhD
Project Description
Small secondary ovarian follicles are capable of growing in a 3D alginate matrix to generate fertilizable eggs that can give rise to live offspring in mice. Though this system has been proven successful, the percentage of competent oocytes generated from each pool of cultured follicles remains low. It is known that somatic cell-oocyte interactions, namely via transzonal projections (TZPs), are necessary for proper follicle growth and oocyte development, and that these interactions can be compromised during prolonged follicle culture. Studies have also indicated that GDF9, an oocyte maternal-effect gene, plays a role in maintaining these physical connections between somatic cells and the oocyte. The goals of this pilot study are 1) to evaluate the organization and maintenance of TZPs in growing follicles cultured in our 3D alginate system in mice, primates, and humans and 2) to determine if higher levels of secreted GDF9 correlate with well-organized somatic cell-oocyte interactions, successful follicle growth, and oocyte competency. These studies will elucidate if all follicles have the potential to become dominant follicles producing competent oocytes.
8. Identification of Developmental Markers and Culture Environments
Site: Northwestern University
Principal Investigators: Lonnie Shea, PhD, and Linda Broadbelt, PhD
Project Description
In developing cultures systems for growing follicles, there are at least two significant challenges: i) determining if follicles are developing appropriately within the culture environment, and ii) providing key factors at the appropriate time to promote follicle growth and maturation. In this pilot project, we will analyze microarray data obtained from mouse ovarian follicles at different stages to identify factors that vary in expression levels between stages and can, therefore, be used as markers of growth progression. We will also use bioinformatics tools to identify key biological pathways that are active during the transition between the multiple follicle stages. This analysis will assist us in identifying factors that could be added to the culture medium to promote follicle growth at specific stages. Results from these studies will be translated to the culture of nonhuman primate and human follicles. The strategy developed in this proposal will provide a foundation for identifying environments that maximize the growth and development of immature follicles and will identify mechanisms through which the environment influences follicle maturation.
9. Role of GDF-9 in Human Folliculogenesis
Site: University of California, San Diego
Principal Investigator: Shunichi Shimasaki, PhD
Co-Investigator: Heidi Cook-Andersen, PhD
Project Description:
Genetic studies in female mice and ewes have demonstrated that the oocyte-specific growth and differentiation factor-9 (GDF-9) regulates female fertility. In humans, a high incidence of mutations in the GDF-9 gene has been found in women with premature ovarian failure (POF) and mothers of dizygotic (DZ) twins. In addition, GDF-9 mRNA levels are reduced in growing human oocytes in polycystic ovary syndrome (PCOS) and PCO ovaries from primordial follicle recruitment through the small Graafian follicle stage. Despite these important clinical observations, almost nothing is known about the mechanism by which GDF-9 controls ovarian function in women. In this pilot project, we will use our purified recombinant human GDF-9 (rhGDF-9) to determine the biological function of the rhGDF-9 in follicle growth and development in human ovaries. We will also determine the biological function of the mutant GDF-9 proteins described in POF women and mothers of DZ twins. The results of these studies will provide the first insight into understanding the molecular and cellular mechanisms of how ovarian function in women is governed physiologically by GDF-9 during reproductive years.
10. Does Gleevac Block Programmed Cell Death in Primary and Primordial Mouse Follicles Via the p63 Pathway?
Site: Northwestern University, Feinberg School of Medicine
Principal Investigator: Teresa K. Woodruff, PhD
Co-Investigator: Takeshi Kurita, PhD, Lonnie D. Shea, PhD, So-Youn Kim, PhD
11. Gene Expression in Small Antral Follicles Derived in Vivo and From Encapsulated 3-Dimensional Culture in Macaques
Site: Oregon National Primate Research Center
Principal Investigator: Mary B. Zelinski, PhD
Co-Investigators: Richard L. Stouffer, PhD, and Jing Xu, PhD
12. Comparing Infertility and Cancer Research, Development and Social Understandings
Site: Northwestern University, Center for Bioethics
Co-Investigator: Lisa Campo-Engelstein, PhD, and Sarah Rodriguez, PhD
13. The Road to Adulthood: How Do Young Adults Make Health and Fertility-Related Decisions
Site: Northwestern University, Feinberg School of Medicine
Principal Investigator: Karrie Snyder, PhD
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and UL1DE019587.
The Patient-Physician Interface: How Breast Cancer Patients Navigate Fertility Concerns and Treatment Options
Site: Northwestern University
Principal Investigator: Karrie Snyder, PhD
Overview
This project assesses how adult women with breast cancer share their concerns regarding infertility with their physicians, how they arrive at a treatment plan with their doctor, and the impact of possible infertility on their lives and relationships after cancer, with special attention being paid to issues of diversity in patient’s concerns and survivorship experiences.
Objectives
- Understand how adult women diagnosed with cancer and their physicians discuss fertility issues and make treatment choices, and how fertility impacts women’s lives after cancer.
- Understand the process of patient-physician decision-making on a broader level to aid in unearthing possible inequities across groups of female cancer patients in terms of access to information regarding infertility, ability to pursue fertility preservation and later fertility treatments, and the resulting differences in life experiences, personal relationships, and family plans.
Indices of Scholarship
- Recruit a sufficient number of women diagnosed with breast cancer (within the past 2 years) and physicians who treat younger cancer patients for in-depth interviews to gain a better understanding of the concerns of women and their relationship with their physicians
- Conduct patient interviews composed of questions related to demographic and illness information, treatment decisions and relationship with the doctor, family/intimate relationships, and issues of identity and gender roles
- Analyze interview data and conduct follow-up interviews (2-3 years later) to track how women’s fertility concerns and family/partnership plans and goals change over time and how infertility-related health care decisions have impacted their lives
Consortium Support and Impact
This project draws on other parts of the Oncofertility Consortium in developing a social science inquiry that is informed by scientific innovation and insight. It relies on clinical and medical perspectives and advances and application of oncofertility technology (see Follice Cryopreservation, Bioengineering Primate Follicles, and Human Follicle Maturation In Vitro) to develop surveys, focus group guidelines, and interviews while serving as an integrated link to interdisciplinary biomedical research in ovarian follicle harvesting, cryopreservation, maturation, and fertilization. Insights regarding the psychosocial impact of infertility on the lives of cancer patients and their families will be applied to the training, education, and advocacy mission of the Consortium.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058296.
Training Global Scholars
Site: University of Pennsylvania
Principal Investigator: Christos Coutifaris, MD, PhD
Rationale
In recent years, cancer diagnosis has changed, in many cases, from a death sentence to a disease with many survivors. Aggressive cancer treatments, although successful with respect to survival, may result in damage or loss of oocytes and impairment or loss of fertility. The purpose of the Oncofertility Consortium is to address the issue of fertility preservation in cancer patients with sufficiently trained physician-scientists who will implement the research and clinical agenda required to treat their patients with new and emerging technologies.
This multidisciplinary force of oncofertility specialists, specifically equipped to cope with the fertility concerns of patients with cancer, represents a new discipline in the field of Women’s Health. Long-term success will depend on how well the development of this global workforce is orchestrated, supported, and focused. It is the intent of this project to train and educate the first generation of oncofertility specialists in research related directly to the reproductive needs of the cancer patient and survivor. Education and training programs will facilitate the development of the oncofertility discipline that exists at the intersection of oncology, pediatrics, reproductive science and medicine, biomechanics, material science, mathematics, social science, bioethics, religion, policy research, reproductive health, law, and cognitive and learning science.
Specifically, this program will train and fund both NRSA eligible and non-NRSA eligible trainees through 2 different NIH grant mechanisms. Oncofertility training will merge entirely with the clinical and education objectives of the fellowship training program, Reproductive Endocrinology and Infertility (REI). Therefore, the likely source of oncofertility specialists will come directly from within the ranks of the REI subspecialty fellowships for advance training after completion of a residency in Obstetrics and Gynecology.
The objectives of this program are to:
- Expand the training of oncofertility specialists within REI programs across the nation through a rigorous educational, laboratory-based, and research curriculum.
- Seek federal funding for additional training dedicated to laboratory work and research related to oncofertility.
Indices of Scholarship
- Train fellows in cellular and molecular approaches to reproductive biology relevant to oncofertility and the responsible conduct of research
- Require the completion of additional didactic training and an online educational module
- Provide clinical exposure to adult and pediatric oncology patients who face the prospect of fertility loss or compromise
- Participate in an academic enrichment program consisting of seminars, lecture series, journal clubs, conferences, and retreats geared towards a comprehension of the interdisciplinary nature of oncofertility
Trainees To Date
- Dr. Monica Mainigi trained at Northwestern in the isolation and culture of early and secondary mouse follicles. The objectives of her research entitled “Developmental Competence of the Mammalian Oocyte: Studies Utilizing an in vitro Model of Follicular Maturation” were (1) To compare the nuclear and developmental competence of oocytes grown at physiologic oxygen (5%) versus atmospheric oxygen (20%) in a three dimensional follicle system, (2) to investigate to effect of in vitro follicle culture on gene expression in the fully grown oocyte and (3) to determine if the culture of immature follicles in vitro leads to disruption of events necessary for successful oocyte activation and fertilization.
- Dr. Fanzhen Hong trained at the University of Pennsylvania in the isolation and culture of early and secondary mouse follicles. Dr. Hong’s main laboratory training initiative was to gain expertise in the isolation and culture of mouse preantral follicles and participate in all the didactic initiatives of the Division of Reproductive Endocrinology and Infertility. Arrangements are currently being made between our program and her University to provide the necessary guidance for setting up a clinical Oncofertility program in the Province of Shandong in China.
- Dr. Shiying Jin trained and gained expertise in the isolation and culture of early secondary mouse follicles at Northwestern University. Dr. Jin’s focus during the training period was to gain expertise in the isolation and culture of mouse preantral (early secondary) follicles and initiate studies to optimize the cryopreservation and in vitro culture system for the culture of human follicles.
- Dr. Jayon Kim is training at the University of North Carolina, Chapel Hill with Dr. Jennifer E. Mersereau. Dr. Kim is involved in a variety of studies including trials evaluating the efficacy of oocyte cryopreservation; the complex relationships between BRCA mutations, breast cancer, and reproductive hormones, and more. Read one of her recently published papers.
- Additional trainees will be accepted annually as the program continues
Consortium Support and Impact
This project will draw on the biomedical and clinical research of the Oncofertility Consortium. Fellows will be trained in cryopreservation techniques and storage, follicle growth research in primates, the effects of cancer treatment on women’s reproductive capacities and the growth and maturation of human follicles, and tissue engineering applications. Each fellow will be expected to receive instruction in bio-psychosocial science, bioethics and religion, and reproductive health policy and law related to oncofertility. Online Resources will provide additional information to those being trained to ensure the optimal educational environment. Currently, there are 3 projects (see Fertility Measures After Cancer and Pilot Project Program) funded through the Consortium and being led by members of the first generation of oncofertility specialists, who use and provide feedback on the education and training program.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and TL1CA133837/RL9CA133838.
Understanding Psychological Adjustment and Decision Making in Advanced Cancer
Site: Northwestern University
Principal Investigators: Linda Emanuel, MD, PhD; Maxwell Vergo, MD; Lynne Wagner, PhD
Overview
Our immediate goal is to provide a foundation to understand processing and decision making in new cancer patients when a single immediate decision about oncofertility must be made. Our long terms goals include improving the quality of cancer survivorship through appropriately supported patient-centered care decisions and reassessing how informed consent can be most valid, in light of how human processing occurs.
Objectives
- To establish a feasible method to collect qualitative and quantitative data in real time, focusing on cognition, affect, and behavior relevant to oncofertility decisions made after the diagnosis of a new cancer.
- To examine processes of psychological adjustment and influences on a fertility preservation decision occurring in the context of a new cancer diagnosis.
- To accumulate evidence on the validity of two related conceptual models of adjustment to serious life changes such as receiving a cancer diagnosis
Indices of Scholarship
- Conduct initial interviews and train patients in completing quantitative and qualitative assessments.
- Ensure that participants complete of daily logs and voice recordings of qualitative and quantitative probes. The daily assessment will include a brief narrative description of losses that occurred or decisions that were made in the previous 24 hours.
- Conduct descriptive analyses of compliance rates, accural rate, and satisfaction and examine predictors of compliance according to clinical and socioeconomic variables.
- Characterize processes of psychological adjustment to losses.
Consortium Support and Impact
This project draws on other parts of the Oncofertility Consortium in developing a social science inquiry that is informed by scientific innovation and insight. It relies on clinical and medical perspectives and advances and application of oncofertility technology (see Follice Cryopreservation, Bioengineering Primate Follicles, and Human Follicle Maturation In Vitro) to develop surveys, focus group guidelines, and interviews while serving as an integrated link to interdisciplinary biomedical research in ovarian follicle harvesting, cryopreservation, maturation, and fertilization. Insights regarding the psychosocial impact of infertility on the lives of cancer patients and their families will be applied to the training, education, and advocacy mission of the Consortium.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058296.
Waiting to be Born: The Ethical and Legal Implications of the Generation from Frozen and Stored Pre-pubertal Ovarian Tissue
Site: Northwestern University
Principal Investigators: Linda Emanuel, PhD, MD; Sherman Elias, MD; Laurie Zoloth, PhD; and Dorothy Roberts, JD
Overview
This project evaluates the ethical and legal issues surrounding the use of reproductive technologies in cancer patients, with a special emphasis on the implications for pediatric patients and their families.
Objectives
- A critical assessment of the ethical, religious, and legal constraints associated with the application of advanced fertility opportunities to young women and girls facing fertility-threatening diseases.
- An evaluation of the existing norms of pediatric decision-making for the young child and family who will make such decisions on their behalf, and the implications for medical practice and health care policy.
- An assessment and consideration of the competing moral appeals that arise in connection with the long-term uses of this research.
Indices of Scholarship
- Meet with leaders from religion, law, psychology, social science, political science, and biomedical policy to identify all the major issues raised by the advanced fertility options in this project
- Based on the issues identified, conduct a comprehensive literature review that will result in a manuscript to set out the issues, identify arguments, and highlight the areas in need of further deliberation
- For each key issue, identify real and hypothetical, but realistic, cases for analysis of: (1) medical risks and benefits; (2) how best to communicate with patients and families on issues of oncofertility; (3) economic considerations; (4) ethical options; and (5) decision-making approaches for clinicians, patients, and families
- Reconvene at a mini-symposium to discuss positions, fill gaps, and collectively author a consensus paper that will provide direction and make recommendations regarding the professionally-guided ethical norms of practice for advanced reproductive oncofertility technology. Articles, notes, and presentations from the Center for Bioethics, Science and Society’s Oncofertility Summit are available in the Virtual Library.
Consortium Support and Impact
This project draws on other parts of the Oncofertility Consortium in developing a social science inquiry that is informed by scientific innovation and insight. It relies on clinical and medical perspectives and advances and application of oncofertility technology (see Follice Cryopreservation, Bioengineering Primate Follicles, and Human Follicle Maturation In Vitro) to develop surveys, focus group guidelines, and interviews while serving as an integrated link to interdisciplinary biomedical research in ovarian follicle harvesting, cryopreservation, maturation, and fertilization. Insights regarding the psychosocial impact of infertility on the lives of cancer patients and their families will be applied to the training, education, and advocacy mission of the Consortium.
Publications
Backhus LE and Zoloth L. Today’s Research, Tomorrows Cures: The Ethical Implications of Oncofertility. 2007.
Zoloth L, Backhus LE and Woodruff TK. Waiting to be Born: The Ethical Implications of the Generation of “NUBorn” and “NUAge” Mice from Pre-Pubertal Ovarian Tissue. The American Journal of Bioethics, 8:6,21 — 29, 2008.
Zoloth L, Backhus LE Woodruff TK, Henning A, and Raucher M. Like/As: Metaphor and Meaning in Bioethics Narrative. The American Journal of Bioethics, 8(6): W3-W5, 2008.
Dolin G,. Roberts DE, Rodriguez LM, Woodruff TK. Medical hope, legal pitfalls: Potential legal issues in the emerging field of oncofertility. Santa Clara L. Rev. 2009.
Zoloth, L. Jewish Perspectives on Oncofertility: The Complexities of Tradition. Cancer Treatment and Research. 2010; 156: 307-17. PMID: 20811844.
Zoloth, L. Final Thoughts. Cancer Treatment and Research. 2010; 156: 487-9. PMID: 20811861.
Zoloth, L and Henning A. Bioethics and Oncofertility: Arguments and Insights from Religious Traditions. Cancer Treatment and Research. 2010; 156: 261-78. PMID: 20811840.
This research was supported by the Oncofertility Consortium®, funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant UL1DE19587 and RL1HD058296.

