Systemic Lupus Erythematosus (SLE)
| Prevalence | Lupus affects approximately 1.5-2 million Americans and is ten times more common in women than in men. In fact, >90% of Americans diagnosed with Lupus are women and it is estimated that one in every 2000 women have Lupus[1]. |
| Natural History | Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease with characteristic exacerbations and remissions that affects multiple organ systems [2]. It can affect the skin, joints, kidneys, brain, and other organs. The underlying cause is unknown. |
| Disease Presentation | 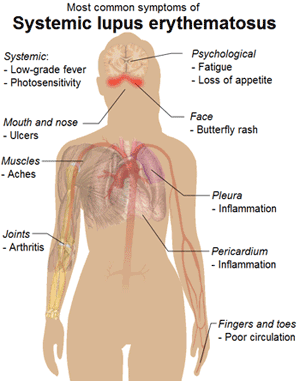 The symptoms of SLE[2,3] include: The symptoms of SLE[2,3] include:
Other symptoms depend on which part of the body is affected.
|
| Effect on Female Fertility | Menstrual Disturbances
Many women with SLE experience menstrual disturbances ranging from amenorrhoea to menorrhagia[4]. Women who receive anti-coagulation therapy for thrombotic complications may experience menorrhagia. Amenorrhoea may occur as the result of cyclophosphamide (CYC) treatment, which can cause ovarian failure, but also as a result of the disease itself. Amenorrhoea in women with SLE is also associated with anti-corpus luteum antibodies with raised FSH levels, suggestive of an autoimmune-SLE related menstrual dysfunction[5]. Pasoto et al. (2002) found 53% of adult women under the age of 40 years with SLE had menstrual irregularity in the absence of alkylating agents[6]. Those with high disease activity were found more likely to have menstrual disturbances than those with less disease activity Reproductive System Impairment Impairment of the reproductive system of women with SLE may result due to disease activity, autoantibodies, and through iatrogenic cytotoxic treatments[7-8]. Impairment of the reproductive system may cause:
Drug-Related Infertility Despite improvements in the treatment options for SLE, the disease can be therapeutically challenging for rheumatologists. Patients with acute exacerbations with severe organ manifestation may need cyclophosphamide (CYC). CYC is an alkylating chemotherapeutic agent shown to cause premature ovarian insufficiency (POI) and deplete healthy oocytes[7,9]. It’s toxicity is associated with many secondary risk factors, including: the cumulative dose of CYC, the age at which the drug is administered, history of thyroid disease, disease duration, and presence of anti-Ro and anti-U1RNP antibodies[7,9-10]. Therefore, CYC should be prescribed at the lowest effective dose possible and for the shortest duration, particularly in older women. Boumpas et al. (1993) found that 17% of patients under age 25 years with > 15 cycles of CYC developed POI. Of those over the age of 30 years, 100% developed POI[11]. Antiphospholipid Antibody syndrome (APS) Approximately 30% of women with SLE also have Antiphospholipid Antibody syndrome (APS), or Hughes’ syndrome. Traditionally, APS is known for causing spontaneous abortions, stillbirths, and premature births. However, APS can lead to venous and arterial thrombotic events, which may restrict the blood supply to reproductive organs, thereby affecting fertility. APS should be considered a risk factor for infertility in women with SLE[4]. Lupus Nephritis Approximately 30-75% of women with SLE, dependent on ethnic and racial background, develop Lupus nephritis- chronic renal failure. Women with chronic renal failure are likely to develop infertility through hypothalamic-pituitary dysfunction, because renal failure and/or haemodialysis tend to raise prolactin levels- which reduces the production of gonadotropin releasing hormone (GnRH) from the hypothalamus. Women with Lupus nephritis tend to have menstrual irregularity with anovulatory cycles[4]. |
| Effect on Male Fertility | Reproductive System Impairment
Impairment of the reproductive system in men with SLE may result due to disease activity, autoantibodies, and through iatrogenic cytotoxic treatments[7-8]. Impairment of the reproductive system may be due to problems preventing successful fertilization, thus leading to infertility, such as testicular failure and sperm abnormalities. The degree of sperm abnormalities has been correlated with a significant reduction in testes volume in men with SLE, as determined by ultrasound imaging. This is most likely due to damage to the seminiferous tubules[12]. Drug-Related Infertility Despite improvements in the treatment options for SLE, the disease can be therapeutically challenging for rheumatologists. Patients with acute exacerbations with severe organ manifestation may need cycylophosphamide (CYC). CYC is an alkylating chemotherapeutic agent shown to impair spermatogenesis. In boys treated with post-pubertal CYC therapy, sperm quantity and quality were significantly impaired compared to health controls. CYC is associated with reduced Leydig cell count and reduced testosterone levels[4]. Methotrexate (MTX) and sulfasalazine (SSZ) can also reduce sperm count and may cause infertility in males[4]. Autoimmune orchitis Approximately 40% of men with SLE have autoimmune orchitis, which often causes male infertility and is characterized by the presence of specific anti-sperm antibodies (ASA), with limited sperm abnormalities[13]. Men with SLE are fourteen times more likely to have Klinefelter’s syndrome than healthy males[14]. Men with Klinefelter syndrome have low testosterone with high FSE and LH levels and are typically infertile. Fertility may be restored in these men by surgical removal of sperm and IVF therapy. |
| Fertility Preservation Options for Women | Gonadotrophic Receptor Hormone Agonist
Leuprolide, a gonadotrophic receptor hormone (GnRH) agonist, is protective against POI when administered 10-14 days before each CYC pulse. Leuprolide causes a drastic reduction in oestrogen and progesterone levels, which significantly reduces the risk of POI in patients with SLE from 30% to 5%[15].
Ooctyte and embryo cyropreservation are the most effective methods to preserve fertility, however in women with SLE hormonal manipulation may trigger exacerbation of SLE as a result of raised oestrogen levels following ovarian stimulation. Exogenous oesterogen therapy is also associated with an increased risk of thrombosis. The safest approach is to avoid overt ovarian hyperstimulation syndrome (OHSS) and administer co-adjuvant therapy and use of natural E(2) or P through non-oral route[7]. Ovarian Tissue Cryopreservation One entire ovary is removed surgically and the outer surface (cortex), which contains the eggs, is frozen in strips for later use. Women who have underwent CYC can have pieces of the tissue thawed and transplanted back. A number of pregnancies have resulted from using this technique. |
| Fertility Preservation Options for Men |  Sperm Banking Sperm Banking
Sperm cells are collected and frozen for future use. Testicular Tissue Banking Testicular tissue, including cells that produce sperm and sperm itself, is removed and frozen. The procedure has varying risks and side effects. This options may not be appropriate for many patients. Testosterone Testosterone can be administered to maintain spermatogenesis in men undergoing CYC treatment for SLE. Studies have shown that 100mg i.m. fortnightly can preserve fertility while on CYC[16]. The testosterone is effective at preserving fertility because it reduces endogenous gonadotrophin release. |
| Fertility Preservation Expert Opinion | Henes et al. (2012)
SLE patients are young and have an excellent response to treatment as well as long-term survival. The Henes et al. (2012) FertiPROTEKT study enrolled 68 women with SLE, with an average patient age of 25 and 91.2% of participants did not have children. Of the 68 women enrolled, only 5 chose not to preserve fertility prior to CYC treatment. Of those who did elect to preserve fertility, 91.2% had GnRH analogue treatment and 25% cryopreserved ovarian tissue, of which 4.4% retrieved and froze oocytes. Henes et al. (2012) made fertility preservation recommendations for women with SLE based on study results and literature review:
|
References
[1] The Lupus Foundation of America
[2]Hirshfeld-Cytron, J., Gracia, C., & Woodruff, T. K. (2011). Nonmalignant diseases and treatments associated with primary ovarian failure: an expanded role for fertility preservation.J Womens Health (Larchmt), 20(10), 1467-1477. doi: 10.1089/jwh.2010.2625
[3] Systemic lupus erythematosus. 2014. Medline Plus. http://www.nlm.nih.gov/medlineplus/ency/article/000435.htm
[4]Hickman, R., & Gordon, C. (2011). Causes and management of infertility in systemic lupus erythematosus. Rheumatology, 50(9), 1551-1558
[5]Pasoto, S., Viana, V., Mendonca, B., Yoshinari, N., & Bonfa, E. (1999). Anti-corpus luteum antibody: a novel serological marker for ovarian dysfunction in systemic lupus erythematosus? J Rheumatol, 26, 1087-1093
[6]Pasoto, S., Mendonca, B., & Bonfa, E. (2002). Menstrual disturbances in patients with systemic lupus erythematosus without alkylating therapy: clinical, hormonal, and therapeutic associations. Lupus, 11, 175-180
[7]Hickman, R., & Gordon, C. (2011). Causes and management of infertility in systemic lupus erythematosus. Rheumatology, 50(9), 1551-1558.
[8]Velarde-Ochoa, M., Esquivel-Valerio, J., Vega-Morales, D., Skinner-Taylor, C., Galarza-Delgado, D., & Garza-Elizondo, M. (2014). Anti-mullerian hormone in reproductive age women with systemic lupus erythematosus. Reumatol Clin.
[9]Mok, C., Lau, C., & Wong, R. (1998). Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum, 41, 831-83
[10]Henes, M., Henes, J., Neunhoeffer, E., Von Wolff, M., Schmalzing, M., Kotter, I., & Lawrenz, B. (2012). Fertility preservation methods in young women with systemic lupus erythematosus prior to cytotoxic therapy: experiences from the FertiProtekt network. Lupus, 21(9), 953-958.
[11]Boumpas, D., Austin, H. r., Vaughan, E., Yarboro, C., Klippel, J., & Balow, J. (1993). Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med, 119, 366-369.
[12]Soares, P., Borba, E., Bonfa, E., Hallak, J., Correa, A., & Silva, C. (2007). Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum, 56, 2352-2361.
[13]Carp, H. J., Selmi, C., & Shoenfeld, Y. (2012). The autoimmune bases of infertility and pregnancy loss. J Autoimmun, 38(2-3), J266-274.
[14]Scofield, R., Bruner, G., Namjou, B., Kimberly, R., Ramsey-Goldman, R., Petri, M., . . . Harley, J. (2008). Klinefelter’s syndrome (47, XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum, 58, 2511-2517.
[15]Somers, E., Marder, W., Christman, G., Ognenovski, V., & McCune, W. (2005). Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum, 52, 2761-2767.
[16]Masala, A., Faedda, R., Alagna, S., Satta, A., Chiarelli, G., Rovasio, P., . . . Bartoli, E. (1997). Use of Testosterone To Prevent Cyclophosphamide-Induced Azoospermia. Ann Intern Med, 126(4), 292-295
About the Author
Allison Goetsch, MS is a genetic counselor in Northwestern University Feinberg School of Medicine’s Department of Obstetrics and Gynecology. She completed her graduate thesis with Dr. Teresa K. Woodruff on oncofertility and hereditary breast and ovarian cancer (HBOC) syndrome and is now a member of the Oncofertility Consortium® administrative core team. Allison’s primary goals are to increase fertility preservation awareness and education for both health care providers and patients regarding the impact malignant and non-malignant diseases (and/or treatments) may have on fertility.
This page was last updated June 5th, 2014.


 Oocyte or Embryo Cryopreservation
Oocyte or Embryo Cryopreservation