Aspartylglucosaminuria
| Incidence |  Aspartylglucosaminuria (AGU) (OMIM #208400) occurs worldwide, but is primarly found in individuals of Finnish ancestry[1]. AGU affects approximately 1 in 3,643 children in Finland and approximately 1 out of every 30 Finnish individuals are carriers of the autosomal recessive condition[2]. Aspartylglucosaminuria (AGU) (OMIM #208400) occurs worldwide, but is primarly found in individuals of Finnish ancestry[1]. AGU affects approximately 1 in 3,643 children in Finland and approximately 1 out of every 30 Finnish individuals are carriers of the autosomal recessive condition[2]. |
| Natural History | AGU is a rare, inherited lysosomal disease caused by mutations in AGA. AGA encodes the enzyme aspartylglucosaminidase (AGA). AGA is active within the lysosomes, where it helps break down complexes of sugar molecules (oligosaccharides) attached to glycoproteins. Mutations in AGA cause an absence or shortage of AGA enzyme in the lysosomes, preventing the break down of oligosaccharides resulting in a build-up of glycoproteins that disrupt normal cell function, often resulting in cell destruction. Nerve cell destruction in the brain causes many of the signs and symptoms of AGU, including progressive mental retardation[3]. |
| Disease Presentation | 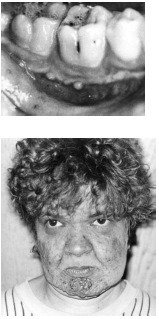 Infants with AGU appear healthy at birth and typically have normal development throughout early childhood. The first sign of the disease is usually delayed speech, which appears between ages 2 and 3 years. As the children age, a mild intellectual disability becomes apparent and learning occurs at a slower than normal pace. Children with AGU are prone to upper respiratory infections and are tall for their age. They have a characteristic facial appearance with ocular hypertelorism, small ears, full lips, short and broad nose, a square-shaped face, and overgrown oral mucosa and facial skin. Infants with AGU appear healthy at birth and typically have normal development throughout early childhood. The first sign of the disease is usually delayed speech, which appears between ages 2 and 3 years. As the children age, a mild intellectual disability becomes apparent and learning occurs at a slower than normal pace. Children with AGU are prone to upper respiratory infections and are tall for their age. They have a characteristic facial appearance with ocular hypertelorism, small ears, full lips, short and broad nose, a square-shaped face, and overgrown oral mucosa and facial skin.
In adolesence, the intellectual disability usually worsens. A maximal intellectual level and adaptive skills of a 5-to-6 year old child is reached between 13 and 16 years of age before a decline of mental abilities occurs. Puberty occurs early in individuals with AGU- girls experience menarche at an average age of 10 years and young boys have macroorchidism. Adolescents with AGU do not experience a growth spurt during puberty and therefore tend to have short stature. Adults with AGU have profound mental retardation with a limited vocabularly. They tend to be quiet and calm, however when disturbed they can be aggressive. Approximately 25% of adults with AGU experience behavioral disturbances. After age 25-28 years, rapid mental and physical retardation occurs with death occuring on average in mid-adulthood[1,4,5]. |
| Effect on Fertility | Hematopoietic stem-cell transplantation (HSCT) can provide the deficient enzyme AGA to individuals with AGU. The outcome of HSCT depends on how rapid the natural disease progression occurs, at what age the HSCT is performed, and the influence of transplant-related complications; therefore, HSCT should not be encouraged for the treatment of patients with AGU after infancy to increase the likelihood of a successful outcome[6].
Malm et al. (2004) conducted a five year follow-up after two siblings with AGU underwent HSCT using unrelated human leukocyte antigen-A, -B, and DR identical donors at age 10 years 5 months and 5 years 10 months, respectively. After 5 years no neuropsychologic or clinical deterioration was noted in either sibling. Additionally, both siblings had stable expression of AGA. Therefore, HSCT may arrest disease progression and could contribute to a better quality of life in individuals with AGU. Arvio et al. (2001) described 19 young patients with AGU. Of the 19 individuals, 5 had undergone successful bone marrow transplantation between 1991 and 1997. Two patients who received transplants were more severely mentally retarded 7 and 5 years’ follow-up than the nontransplanted patients. Additionally, all 5 individuals experienced posttransplant complications. Therfore, Arvio et al. (2001) concluded that bone marrow transplantation should not be recommended to patients with AGU after infancy.
Overall, the goal of HSCT is to cure the patient from the primary disease and restore complete physical and mental health condition; however, infertility is common after myeloablative HSCT conditioned with total body irradiation (TBI) and high doses of gonadotoxic drugs[7]. Female Fertility HSCT conditioning often involves TBI and/or gonadotoxic drugs, both of which are associated with a high risk of ovarian failure in females[7,8]. The majority of female patients who are treated with TBI experience gonadal failure. In fact, 10% experience recovery of gonadal function and only 1.3% acheive pregnancy[7]. Vatanen et al. (2013) evaluated long-term ovarian function after allogenic HSCT in childhood and adolescence in 70 prepubertal female tranplant survivors and 22 adult transplant survivors (transplant occuring between 1987-2000; mean age of 9 years; age range 1-19 years). Results showed that 40/70 prepubertal females experienced spontaneous puberty, based onbreast development, and 30/70 experience spontaneous menarche. They found that there was a high risk of ovarian failure with conditioning TBI or Bu-based regimes prior to HSCT and intensive anti-leukemia therapy prior to HSCT, including CRT, decreased the possibility of spontaneous menarche. Male Fertility TBI and gonadotoxic drugs are typically used to condition patients prior to HSCT, both of which lead to absence of spermatozoa. In fact, TBI is the main cause of azoospermia in patients treated with HSCT. This is due to the fact that spermatogenic stem cells are more sensitive to chemotherapy and radiation than later stage germ cells, preventing the generation of newsperm cells[7]. Rovo et al. (2013) conducted a European Group for Blood and Marrow Transplantation (EBMT) large retrospective analysis of men who underwent HSCT and found that 81% of males receiving TBI presented withazoospermia and only 1% had normal sperm counts in follow-up spermanalysis. Anserini et al. (2002) found that azoospermia is less frequent in patients conditioned with busulphan and cyclophosphamide (50%) and uncommon in those treated with cyclophosphamide alone. Suggesting, perhaps, that busulphan and cyclophosphamiade or cyclophosphamide alone may affect fertility less than traditional HSCT conditioning with TBI and gonadotoxic drugs. |
| Fertility Preservation |  Female Fertility Preservation Female Fertility Preservation
The risk of premature ovarian failure (POF) and infertility is very high after HSCT, however fertility issues may not become relevant for transplant survivors until a later timepoint. Therefore, fertility preservation discussions should be discussed with all young women of childbearing age and parents of children facing HSCT[12]. The main fertility preservation options available for women include: embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation. However, given that experts recommend HSCT occur during infancy in individuals with AGU[6], they cannot undergo the necessary ovarian stimulation needed for embryo and oocyte cryopreservation. Therefore, ovarian tissue cryopreservation and autotransplantation should be considered prior to HSCT in infants with AGU. Ovarian Tissue Cryopreservation Alternatively, females with AGU may wish to use donor ooctyes or consider adoption.
Given the risk of azoospermia is very high after HSCT, fertility preservation options should be discussed with all young men of reproductive age and parents of children facing HSCT[7]. Infants with AGU are prepubertal at the time of HSCT and prepubertal males cannot produce semen for cryopreservation. Testicular tissue cryopreservation is currently the only option for prepubertal males at high risk for infertility interested in preserving future fertility[12]. Ginsberg et al. (2014) consented 57 families to testicular biopsy, and 28 children underwent the procedure with 1 experiencing a post-operative side effect. Parents who faced this fertility preservation option at their child’s diagnosis reported they made an informed decision and carefully weighed the risks and benefits of testicular tissue cryopreservation. Testicular Tissue Cryopreservation Alternatively, males with AGU may choose to use donor sperm or consider adoption. |
| Expert Opinion | Tichelli & Rovo (2013):
“Long-term survivors are at risk of late complications interfering with health condition and quality of life after HSCT. Infertility is one among other complications that may occur after transplantation. Today it becomes more evident that patients treated with HSCT are at high risk of permanent infertility. Despite not being a life-threatening complication, infertility is associated with significant psychological distress for the recipient of HSCT and his family. Long-term survivors have a legitimate right of becoming parents of children. Today, there are reasonable options to preserve fertility in patients treated with HSCT. For most late effects that may appear after HSCT, surveillance and preemptive interventions are undertaken during long-term follow-up after transplantation. In contrast, fertility preservation needs to be decided and implemented before starting HSCT. Despite high patient’s interest in issues of fertility, a number of barriers impede clinicians to discuss overtly the risk of infertility and the option for fertility preservation with many patients undergoing HSCT. In addition, providing information and guidance about fertilitypreservation raises a number of ethical and legal considerations.” |
| Patient Fact Sheet | Print the Oncofertility Consortium® Fertility Preservation Patient Fact Sheet on Aspartylglucosaminuria (AGU) to provide to your patients and their families who are considering fertility preservation prior to HSCT treatment. This fact sheet is tailored specifically to the fertility needs of children with Aspartylglucosaminuria (AGU).
Aspartylglucosaminuria Fertility Preservation Patient Fact Sheet |
References
[1] Arvio, P., & Arvio, M. (2002). Progressive nature of aspartylglucosaminuria. Acta Paediatr, 91(3), 255-257.
[2] Mononen, I., Heisterkamp, N., Kaartinen, V., Williams, J. C., Yates, J. R., III, Griffin, P. R., . . . Groffen, J. (1991). Aspartylglycosaminuria in the Finnish population: identification of two point mutations in the heavy chain of glycoasparaginase. Proc. Nat. Acad. Sci, 88, 2941-2945.
[3] Aronson, N. J. (1999). Aspartylglycosaminuria: biochemistry and molecular biology. Biochim Biophys Acta, 1455(2-3), 139-154.
[4] Arvio, M., Peippo, M., Arvio, P., & Kaariainen, H. (2004). Dysmorphic facial features in aspartylglucosaminuria patients and carriers. Clin Dysmorphol, 13(1), 11-15.
[5] Malm, G., Mansson, J., Winiarski, J., Mosskin, M., & Ringden, O. (2004). Five-year follow-up of two siblings with aspartylglucosaminuria undergoing allogeneic stem-cell transplantation from unrelated donors. Transplantation, 78(3), 415-419
[6] Arvio, M., Sauna-aho, O., & Peippo, M. (2001). Bone marrow transplantation for aspartylglucosaminuria: follow-up study of tranplanted and non-transplanted patients. J. Pediat, 138, 288-290.
[7] Tichelli, A., & Rovo, A. (2013). Fertility issues following hematopoietic stem cell transplantation. Expert Rev Hematol., 6(4), 375-388.
[8] Vatanen, A., Wilhelmsson, M., Borgstrom, B., Gustafsson, B., Taskinen, M., Saarinen-Pihkala, U., . . . Jahnukainen, K. (2013). Ovarian function after allogenic hematopoietic stem cell transplantation in childhood and adolescence. Eur J Endocrinol, 170(2), 211-218.
[9] Rovo, A., Aljurf, M., Chiodi, S., S, S., Salooja, N., & G, S. (2013). Ongoing graft-versus-host disease is a risk factor for azoospermia after allogeneic hematopoietic stem cell transplantation. A survey of the late effect working party of the European Group for Blood and Marrow Transplantation.Haematologica, 98(3), 339-345.
[10] Anserini, P., Chiodi, S., Spinelli, S., Costa, M., Conte, N., Copello, F., & Bacigalupo, A. (2002). Semen analysis following allogenic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant, 30(7), 447-451.
[11] Jadoul, P., & Donnez, J. (2012). How does bone marrow transplantation affect ovarian function and fertility? Curr. Opin. Obstet. Gynecol., 24(3), 164-171.
[12] Ginsberg, J., Li, Y., Carlson, C., Gracia, C., Hobbie, W., Miller, V., . . . Kolon, T. (2014). Testicular tissue cryopreservation in prepubertal male children: An analysis of parental decision-making. Pediatr Blood Cancer.
About the Author
Allison Goetsch, MS, CGC is a pediatric genetic counselor at Ann and Robert H. Lurie Children’s Hospital of Chicago and a member of the Oncofertility Consortium administrative core team. She completed her graduate thesis with Dr. Teresa K. Woodruff researching oncofertility and hereditary breast and ovarian cancer (HBOC) syndrome. Allison’s primary goals are to increase fertility preservation awareness and education for both health care providers and patients regarding malignant and non-malignant diseases (and/or treatments) which threaten fertility.
This page was last updated February 6th, 2016.


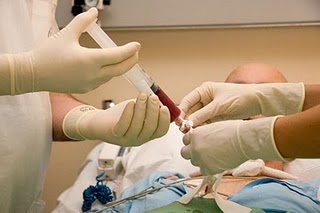 AGU is a rare condition and therefore HSCT use is based on limited safety and efficacy data in humans. The largest experience with HSCT for lysosomal storage diseases exists in Hurler syndrome, for which good long-term neurodevelopmental outcomes have been demonstrated. Therefore, it is possible that early HSCT during infancy may be beneficial in alleviating the disease symptoms and progression of AGU, including neurologic function
AGU is a rare condition and therefore HSCT use is based on limited safety and efficacy data in humans. The largest experience with HSCT for lysosomal storage diseases exists in Hurler syndrome, for which good long-term neurodevelopmental outcomes have been demonstrated. Therefore, it is possible that early HSCT during infancy may be beneficial in alleviating the disease symptoms and progression of AGU, including neurologic function Male Fertility Preservation
Male Fertility Preservation