Ataxia Telangiectasia
| Incidence | Ataxia Telangiectasia (A-T) has a frequency of approximately 1 in 40,000 births in the United States[1].
The carrier rate of A-T, which is an autosomal recessive genetic condition, is approximately 2.8% in the United States[2]. |
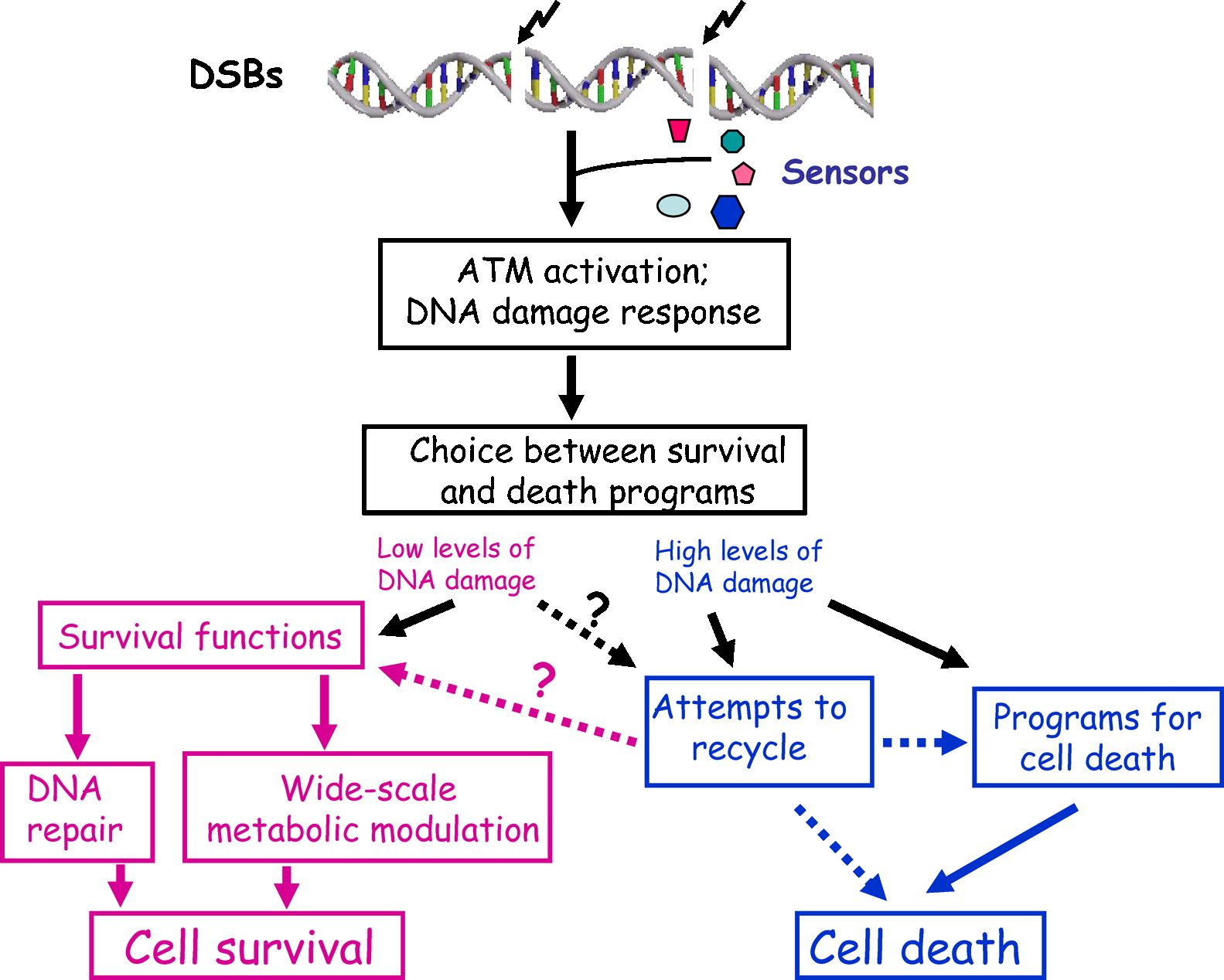
| Natural History |  A-T is an inherited genetic condition caused by mutations in ATM. ATM encodes the ATM protein kinase, which mobilizes cellular response to double strand breaks in DNA by phosphorylating numerous DNA damage response (DDR) players. When both copies of ATM are mutated. the cell’s ability to repair DNA damage is impaired, which leads to degeneration of specific tissues- particularly the nervous and immune systems. It is unknown why absence of ATM protein kinase leads to a slowly progressive disease with a variable phenotype rather than a more severe phenotype, however it may have to do with the redudancy between ATM and its close relative, the protein kinase ATR[3]. A-T is an inherited genetic condition caused by mutations in ATM. ATM encodes the ATM protein kinase, which mobilizes cellular response to double strand breaks in DNA by phosphorylating numerous DNA damage response (DDR) players. When both copies of ATM are mutated. the cell’s ability to repair DNA damage is impaired, which leads to degeneration of specific tissues- particularly the nervous and immune systems. It is unknown why absence of ATM protein kinase leads to a slowly progressive disease with a variable phenotype rather than a more severe phenotype, however it may have to do with the redudancy between ATM and its close relative, the protein kinase ATR[3].
|
| Disease Presentation | A-T has a complex and variable phenotype that affects several body systems and tissues. The primary characteristics of A-T include:
Neurological Symptoms: A-T is characterized by progressive neurodegeneration- primarily affecting the cerebellum. When a child with A-T begins to walk (approximately age 2-3 years), truncal ataxia is generally present. The ataxia subsequently spreads to affect the extremeties, followed by speech. By the end of their first decade of life, children with A-T are typically wheelchair bound[1,3]. Mental Status: In most cases, individuals wtih A-T have normal mental status and many complete university-level education[4].
Immunodeficiency: Individuals with A-T usually develop primary immunodeficiency, affecting the humoral and cellular systems of the body. Immunodeficiency causes a predisposition to infections, particularly sinopulmonary infections[1,3]. Cancer Predisposition: Approximately 1/3 of individuals with A-T develop a malignancy- typically a lymphoma or leukemia. Younger children tend to develop common acute lymphocytic leukemia, while older patients are prone to nonlymphoid cancers, such as: stomach, breast, basal cell, ovarian, liver, uterine, and melanoma. The predisposition to develop malignancies is complicated by the fact that conventional doses of radiation therapy and chemotherapy with radiomimetic agents are associated with adverse and sometimes fatal reactions in individuals with A-T[5]. Sensitivity to Radiation: Individuals with A-T are sensitive to the cytotoxic effect of ionizing radiation, which leads to chromosomal instability[3]. Secondary Complications: The secondary complications of progressive ataxia include deconditioning/immobility, weight loss or gain, skin breakdown, recurrent pulmonary and urinary tract infections, aspiration, occult respiratory failure, and obstructive sleep apnea[4]. Life Expectancy: Life expectancy for individuals with A-T varies, but can be as late as the sixth decade of life. The three main causes of death are malignancy, infection, and nonspecific pulmonary failure[4]. |
| Disease Management | There is no effective way stop the progressive ataxia associated with A-T. Treatment is symptom-based and often includes managing the immunodeficiencies, sinupulmonary infections, neurologic dysfunction, and malignancy. Individuals with A-T usually have physical, occupational, and speech/swallowing therapy.
Medications can be taken to improve balance, coordination, and dysarthria. Supportive interventions, such as disease education, genetic counseling, individual and family counseling, referral to support groups and advocacy groups, as well as guidance to online resources are recommended[4]. |
| A-T Heterozygotes and Cancer Susceptibility | The carrier rate for A-T in the United States is approximately 2.8%[2]. Carriers of A-T have increased cancer susceptibility compared to the general population, although this risk has been debated. Many studies have analyzed the association between cancer risk and A-T heterozygote status.
Morrell et al. (1990) measured the cancer incidence in 574 close relatives of individuals with A-T and 213 spouse controls from 44 previously unreported A-T families. They found that the relative risk of cancer in heterozygous carriers of A-T to be 6.1 compared to the non-carriers. The most frequent site of cancer in the carriers was the breast. Therefore, there may be an increased risk of developing breast cancer in A-T carriers. Athma et al. (1996) analyzed breast cancer diagnoses in the United States and found that 6.6% may occur in women who are A-T carriers. Therefore, A-T carriers may be at greater risk than the general population to develop breast cancer. Easton (1994) found that 8% of breast cancers in women under age 40 years occurs in women who are A-T carriers, compared to 2% of breast cancers in women between age 50 and 59 years. This suggests that A-T carrier status is associated with an increased early-onset breast cancer risk. FitzGerald et al. (1997) findings contradict previous studies by concluding heterozygous ATM mutations (found in A-T carriers) do not increase susceptibility to early onset of breast cancer. However, future studies were in agreement with previous studies, suggesting there is an increased early-onset breast cancer susceptibility with A-T carrier status. Broeks et al. (2000) found the risk of developing breast cancer characterized by frequent bilateral occurrence, early age of onset, and long-term survival is 9-times greater in A-T carriers than the general population. Therefore, women with heterozygous ATM mutations have an increased risk to develop young onset breast cancer and survive following treatment. Renwick et al. (2006) sought to futher quantify the relative risk of developing breast cancer in A-T carrier by screening individuals from 443 familial breast cancer families and 531 controls for ATM mutations. They concluded that ATM mutations do confer breast cancer susceptibilty in A-T carriers with an estimated relative risk of 2.37.
|
| Effect on Fertility | Individuals with A-T are at risk for gonadal dysfunction. The following have all been reported in individuals with A-T:
Zadik, et al. (1978) analyzed gonadal dyfunction in two males and three females diagnosed with A-T between ages 4 and 23 years. All five individuals had elevated basal levels of FSH and three individuals had elevated levels of LH. The pubertal and adult famles had low basal levels of estradiol. The laboratory and clinical findings of all five patients indicated primary gonadal failure is a characteristic of A-T. In addition to risk of gonadal dysfunction, approximately 33% of individuals with A-T develop a malignancy[5]. Gonadotoxic drugs, such as chemotherapy, and/or total body irradiation (TBI) are typically used to treat malignancies, however they may have harmful effects on fertility. |
| Fertility Preservation Options | Parents of children with A-T may be interested in learning about available fertility preservation options for their children who have gonadal dysfunction and/or have been diagnosed with cancer. The available fertility preservation options for children include:
Individuals with A-T who are post-pubertal (or carriers of A-T) and have been diagnosed with cancer may be interested in the following additional fertility preservation options:
|
| Patient Fact Sheet | Print the Oncofertility Consortium® Fertility Preservation Patient Fact Sheet on Ataxia Telangiectasia (A-T) to provide to your patients and their families who are considering fertility preservation due to gonadal dysfunction or cancer diagnosis. This fact sheet is tailored specifically to the fertility needs of patients and families with Ataxia Telangiectasia (A-T).
Ataxia Telangiectasia (A-T) Fertility Preservation Patient Fact Sheet |
References
[1] Chun, H., & Gatti, R. (2004). Ataxia-telangiectasia, an evolving phenotype. DNA Repair, 3(8-9), 1187-1196.
[2] Swift, M., Morrell, D., Cromartie, E., Chamberlin, A., Skolnick, M., & Bishop, D. T. (1986). The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet, 39, 573-583.
[3] Biton, S., Barzilai, A., & Shiloh, Y. (2008). The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst.), 7(7), 1028-1038.
[4] Perlman, S., Becker-Catania, S., & Gatti, R. (2003). Ataxia telangiectasia: a diagnosis and treatment. Semin Pediatr Neurol, 10(3), 173-182.
[5] Swift, M., Sholman, M., Perry, M., & Chase, C. (1976). Malignant neoplasms in the families of patients with ataxia-telangiectasia. Cancer Res, 36(1), 209-215.
[6] Morrell, D., Chase, C., & Swift, M. (1990). Cancers in 44 families with ataxia-telangiectasia. Cancer Genet Cytogenet, 50, 119-123.
[7] Athma, P., Rappaport, R., & Swift, M. (1996). Molecular genotyping shows that ataxia-telangiectasia heterozygotes are predisposed to breast cancer. Cancer Genet Cytogenet, 92, 130-134.
[8] Easton, D. (1994). Cancer risks in A-T heterozygotes. Int J Rad Biol, 66, S177-S182.
[9] FitzGerald, M., Bean, J., Hedge, S., Unsal, H., MacDonald, D., Harkin, D., . . . Haber, D. (1997). Heterozygous ATM mutations do not contribute to early onset breast cancer. Nature Genet, 15, 307-310.
[10] Broeks, A., JHM, U., Floore, A., Dahler, E., Klijn, J., Rutgers, E., . . . van’t Veer, L. (2000). ATM-heterozygous germline mutations contribute to breast cancer-susceptibility. Am J Hum Genet, 66, 494-500.
[11] Renwick, A., Thompson, D., Seal, S., Kelly, P., Chagtai, T., Ahmed, M., . . . Rahman, N. (2006). ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nature Genet, 38, 873-875.
[12] Jones, K. (2006). Smith’s Recognizable Patters of Human Malformation (6 ed.). Philadelpia, PA: Elsevier Saunders.
[13] Zadik, Z., Levin, S., Prager-Lewin, R., & Laron, Z. (1978). Gonadal dysfunction in patients with ataxia telangiectasia. Acta Paediatr Scand, 67(4), 477-479.
About the Author
Allison Goetsch, MS, CGC is a pediatric genetic counselor at Ann and Robert H. Lurie Children’s Hospital of Chicago and a member of the Oncofertility Consortium administrative core team. She completed her graduate thesis with Dr. Teresa K. Woodruff researching oncofertility and hereditary breast and ovarian cancer (HBOC) syndrome. Allison’s primary goals are to increase fertility preservation awareness and education for both health care providers and patients regarding malignant and non-malignant diseases (and/or treatments) which threaten fertility.
This page was last updated February 6th, 2016.


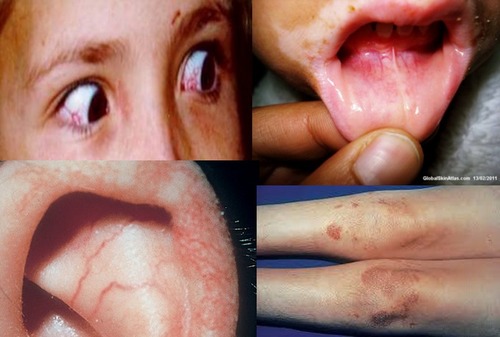 Telangiectasias: Individuals with A-T typically have telangiectasias (dilated blood vessels) which appear variably in the ocular sclerae and occasionally in the facial skin and ears
Telangiectasias: Individuals with A-T typically have telangiectasias (dilated blood vessels) which appear variably in the ocular sclerae and occasionally in the facial skin and ears Due to the increased risk of developing early-onset bilateral breast cancer and increased long-term survival, carriers of A-T may be interested in preserving their fertility prior to cancer treatment or prophylactic cancer risk reducing surgery. It is important to note that many A-T carriers may be identified in absence of family history after the diagnosis of A-T in a child within the family. Genetic counseling is recommended to help explain the implications of A-T carrier status.
Due to the increased risk of developing early-onset bilateral breast cancer and increased long-term survival, carriers of A-T may be interested in preserving their fertility prior to cancer treatment or prophylactic cancer risk reducing surgery. It is important to note that many A-T carriers may be identified in absence of family history after the diagnosis of A-T in a child within the family. Genetic counseling is recommended to help explain the implications of A-T carrier status.